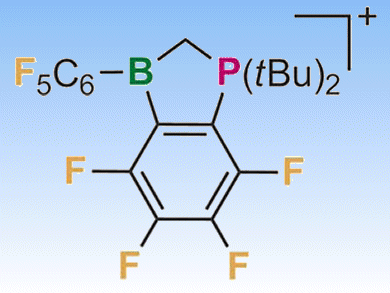
Arylborane Lewis acids find application as homogeneous catalysts. The use of perfluorinated substituents and the incorporation of a positive charge increase their Lewis acidity, improving their properties. This allows the Lewis acid strength to be tailored toward different tasks. The effect of a positive charge on the adduct bond of an anionic Lewis base is an electrostatic interaction and therefore distance-dependent.
Matthias Wagner and colleagues, Goethe-Universität Frankfurt, Germany, synthesized a series of cationic cyclic phosphonium boranes with perfluorinated exocyclic aryl and bridging phenylene ligands. The rigidity of the molecular framework guarantees that the effect of the positive charge does not change over time as the distance remains constant.
These Lewis acids have exceptionally high Gutmann acceptor numbers (AN), form adducts with different uncharged donors (pictured), and catalyze effectively the [4+2] cycloaddition reaction between 2,5-dimethyl-1,4-benzoquinone and cyclopentadiene.
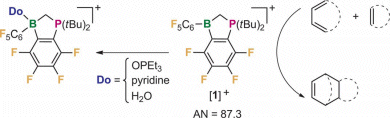
Moreover, replacement of one tBu group in [1]+ by a Me substituent provides access to chiral phosphonium boranes.
- Cyclic Phosphonium Bis(fluoroaryl)boranes – Trends in Lewis Acidities and Application in Diels–Alder Catalysis
A. Schnurr, M. Bolte, H.-W. Lerner, M. Wagner,
Eur. J. Inorg. Chem. 2011.
DOI: 10.1002/ejic.201101098