The introduction of unnatural amino acids (UAAs) into proteins has become a powerful tool for biological studies and creating proteins with novel functions. To date, in vivo UAA incorporation into proteins has been achieved with various organisms, including unicellular organisms such as bacteria and yeast, cultured (single) cells of mammals and of the insect Drosophila melanogaster, and even the multicellular organism Caenorhabditis elegans, a nematode or roundworm.
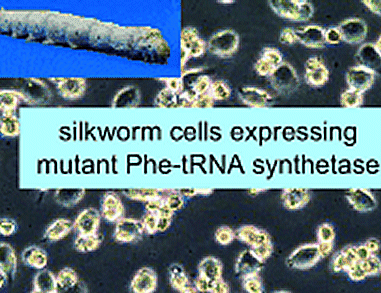
Hidetoshi Teramoto, National Institute of Agrobiological Sciences, Ibaraki, Japan, and colleagues report the extension of UAA incorporation methodology to cultured cells of the domesticated silkworm, Bombyx mori (BmN).
Cultured cells of B. mori were transfected with expression plasmids encoding the aA450G BmPheRS mutant, which
exhibits broader substrate recognition capacity. As a reporter protein, an expression plasmid encoding enhanced green fluorescent protein (EGFP) was also introduced. The cells were cultured with p-Cl- and p-Br-phenylalanine (Phe).
Mass spectrometric analyses of the reporter protein EGFP confirmed the incorporation of p-Cl-Phe in place of native Phe, although no incorporation of p-Br-Phe was observed.
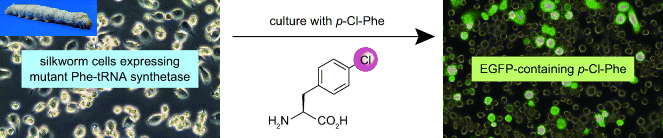
These results demonstrated that the strategy of incorporating UAAs into proteins in a residue-specific manner might be extended to a multicellular animal such as B. mori.
- Expansion of the Amino Acid Repertoire in Protein Biosynthesis in Silkworm Cells,
Hidetoshi Teramoto, Katsura Kojima, Hideyuki Kajiwara, Jun Ishibashi,
ChemBioChem 2011.
DOI: 10.1002/cbic.201100624