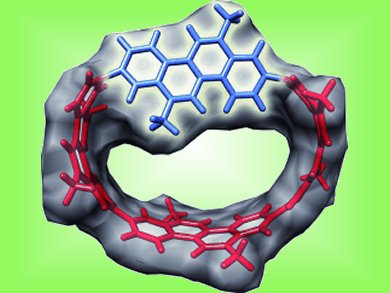
When the rotation around a single bond is strongly hindered, the resulting isolable conformers are called atropisomers. Atropisomerism usually arises from steric hindrance, like in substituted biphenyl or binaphthyl derivatives. A team led by Hiroyuki Isobe, Tohoku University, Sendai, Japan, shows that ring strain can also lead to a sufficiently high barrier to identify atropisomers. This new atropisomerism does not require any steric hinderance from the substituents.
The reseachers constructed a nanohoop molecule from four chrysenylene units, each of which is composed of four fused benzene rings (pictured). They monitored the relative concentrations of the interconverting atropisomers at different temperatures until the mixture equilibrated. From this information, and supported by density functional theory (DFT) calculations, they were able to determine the relative energies of the isomers and gain insight into the thermodynamic behavior of nanocarbon structures.
Future work will investigate the role that substituents play in these processes.
Image: © Wiley-VCH
- Atropisomerism in a Belt-Persistent Nanohoop Molecule: Rotational Restriction Forced by Macrocyclic Ring Strain,
S. Hitosugi, W. Nakanishi, H. Isobe,
Chem. Asian J. 2012.
DOI: 10.1002/asia.201200187