Lasso peptides are an emerging class of ribosomally assembled and post-translationally modified cyclic peptides with a wide range of biological activities. The lasso scaffold features an N-terminal macrocyclic ring composed of 7–9 amino acids and a C-terminal tail that threads through the ring. This structural feature gives high stability against heat treatment and proteases.
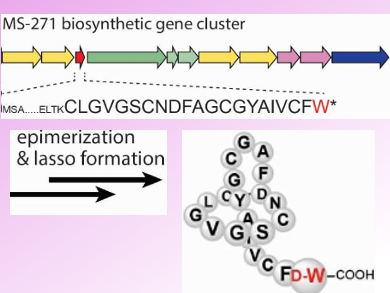
Because of their promising bioactivity and chemical stability, lasso peptides have attracted a lot of attention in recent years. The lasso peptide MS-271 (pictured above) is produced by Streptomyces sp. M-271. It comprises 21 amino acid residues with a D-tryptophan (D-Trp) at its C-terminus. The presence of D-Trp could so far not be explained, as lasso peptides are ribosomally biosynthesized using naturally ocuring L-amino acids.
Yasushi Ogasawara, Tohru Dairi, and colleagues, Hokkaido University, Sapporo, Japan, have identified the MS-271 biosynthetic gene cluster. It is composed of 11 structural genes. In addition, the team showed that the precursor peptide contains all 21 core amino acid residues including the C-terminal D-Trp. This suggests that the D-Trp residue is introduced via an epimerization by a so far unknown enzyme.
The introduction of unnatural D-amino acids into peptides increases their stability. This work laid the foundation for a detailed understanding of the mechanism of this novel peptide epimerization. A detailed understanding s important for redesigning biosynthetic pathways to prepare unnatural peptide secondary metabolites.
- Biosynthetic Gene Cluster of a d-Tryptophan-Containing Lasso Peptide, MS-271,
Zhi Feng, Yasushi Ogasawara, Satoshi Nomura, Tohru Dairi,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800315