Harry L. Anderson, University of Oxford, UK, and colleagues recently developed the concept of Vernier templating—the use of noncommensurate combinations of templates and building blocks to direct the formation of cyclic oligomers, such that the number of binding sites in the product is the lowest common multiple of the numbers of sites in the template and the starting material. This principle allows a small template to direct the formation of a much larger macrocycle.
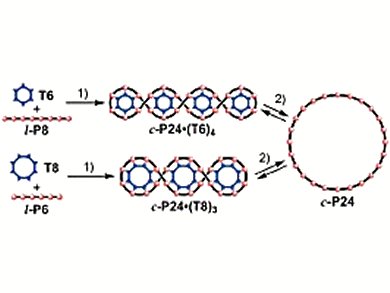
Now they report the synthesis of a 24-porphyrin ring, c-P24, by two Vernier-templated routes (Scheme):
- Coupling the linear porphyrin octamer l-P8 in the presence of hexapyridyl template T6, and
- Coupling the linear porphyrin hexamer l-P6 in the presence of the octapyridyl template T8.
The 24-porphyrin nanoring can be prepared in yields of up to 25 %. It has a diameter of 10 nm, as confirmed by scanning tunneling microscopy (STM), and a molecular weight of 26 kDa, thus placing it in the size range of a typical protein. This flexible macrocycle can be locked into a planar π-conjugated conformation by the cooperative self-assembly of a 2:24 doublestrand complex with the linear diamine ligand 1,4-diazabicyclo[2.2.2]octane (DABCO).
Although there are a few reports of larger π-conjugated macrocycles and larger cyclic porphyrin oligomers, c-P24 is the largest molecular ring to have been synthesized by a template-directed strategy, and this strategy should be applicable to the selective synthesis of even larger macrocycles. The observation that c-P24 can be locked into a planar conjugated tertiary structure by formation of the (c-P24)2•(DABCO)24 sandwich complex indicates that it is a promising system for exploring molecular Aharonov–Bohm effects.
- Two Vernier-Templated Routes to a 24-Porphyrin Nanoring,
Dmitry V. Kondratuk, Luis M. A. Perdigao,Melanie C. O’Sullivan, Simon Svatek, Gareth Smith, James N. O’Shea, Peter H. Beton, Harry L. Anderson,
Angew. Chem., Int. Ed. 2012, 51.
DOI: 10.1002/anie.201202870