Many efforts have been devoted to the capture of CO2 released into the atmosphere. In this context, solution absorption, adsorption, membrane separation, and cryogenic methods have been developed to separate and/or capture CO2. Among these routes, adsorption is of great interest because of its low energy consumption, low equipment cost, and easy application.
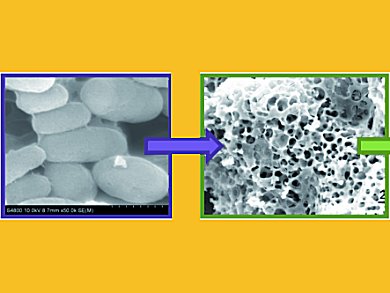
Wenzhong Shen, Weibin Fan and colleagues, Chinese Academy of Sciences, Shanxi, prepared a porous carbon material with a high surface area (1348 m2g–1) and large pore volume (0.67 cm3g–1) by chemical activation of yeast with KOH.
Budding yeast Saccharomyces cerevisiae, a low-cost and widely used microorganism in baking and the ethanol-producing industry, is an elliptical unicellular fungus with a sturdy cell wall mainly composed of carbon, hydrogen, oxygen, and nitrogen atoms; the average nitrogen content is about 10.6 wt%.
The material exhibited high CO2 adsorption capacity as a result of a high content of nitrogen-containing groups and large surface area and pore volume. Its CO2 adsorption amount and rate were much higher and faster than those of the material obtained by directly carbonizing the same yeast.
In particular, the capacity reduction of this adsorbent was less pronounced in the presence of water compared to that of zeolite 13X.
- Yeast-Based Microporous Carbon Materials for Carbon Dioxide Capture,
Wenzhong Shen, Yue He, Shouchun Zhang, Junfen Li, Weibin Fan,
ChemSusChem 2012.
DOI: 10.1002/cssc.201100735