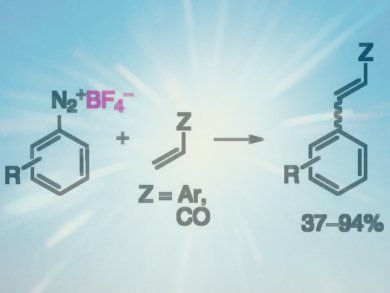
The classical Meerwein arylation uses copper(I) for the arylation of unsaturated compounds with diazonium salts. However, the reaction yields are usually low at high catalyst loadings, and, when performed under aqueous reaction conditions, side products can be formed.
Burkhard König and coworkers, University of Regensburg, Germany, used 2,2′-bipyridine (bpy)-containing ruthenium complexes as catalysts allowing the application of visible light-induced photoredox catalysis. This has been reported to enhance selectivities and reaction rates under mild conditions. The reaction conditions were optimized using phenyldiazonium tetrafluoroborate and styrene to give stilbene with up to 87 % yield.
The scope of aryl diazonium salts and unsaturated compounds was tested and showed that a range of different substituted aryl diazonium salts and a variety of functional groups including aryl halides are tolerated.
The authors propose a mechanistic model for the photoredox arylation of unsaturated compounds using diazonium salts.
- Photocatalytic Arylation of Alkenes, Alkynes and Enones with Diazonium Salts,
Peter Schroll, Durga Prasad Hari, Burkhard König,
ChemistryOpen 2012, 1(3), 130–133.
DOI: 10.1002/open.201200011
ChemistryOpen – the first society-owned, open-access, chemistry journal – is a journal of ChemPubSoc Europe published by Wiley-VCH.