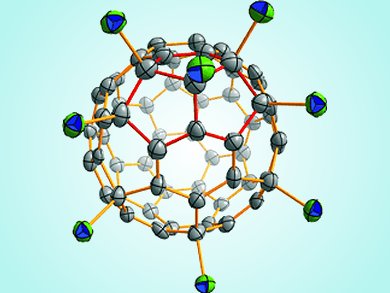
Fullerenes are most stable when all of their pentagons are isolated from each other by hexagons. But how can structures with fused pentagons that share one or even two edges be stabilized?
Su-Yuan Xie and colleagues, Xiamen University, China, used chlorine substituents to change the hybridization of the carbon atoms suffering from pentagon-induced strain from sp2 (with an ideal angle of 120°) to sp3 (with an ideal angle of 109.5°). Uniquely, the X-ray crystal structure of C64Cl8 shows that one of the carbon atoms involved in the triple directly fused pentagon unit is not substituted. This atom, however, participates in a benzoid C6 aromatic system, which also contributes to stabilization of the structure.
This work highlights a new substitution pattern to stabilize fused pentagon units in fullerenes and helps further the understanding of fullerenes that do not follow the isolated-pentagon rule.
Image: © Wiley-VCH
- C64Cl8: A Strain-Relief Pattern to Stabilize Fullerenes Containing Triple Directly Fused Pentagons,
Gui-Juan Shan, Yuan-Zhi Tan, Ting Zhou, Xian-Mei Zou, Bo-Wei Li, Cong Xue, Chen-Xu Chu, Su-Yuan Xie, Rong-Bin Huang, Lan-Sun Zhen,
Chem. Asian J. 2012.
DOI: 10.1002/asia.201200376