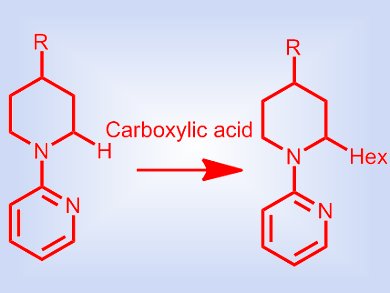
C2-substituted piperidines are highly prevalent structural motifs in natural products and pharmaceuticals. Several direct synthetic approaches have been described, for example, via a C2 radical, anion, or cation intermediate, but all require a stoichiometric reagent. Examples of the direct C2–H functionalization of piperidines by transition-metal catalysis are still rare, although they require less-reactive substrates than the corresponding five-membered cyclic amines.
Bert U. W. Maes and colleagues, University of Antwerp, Belgium, have discovered that carboxylic acid additives improve the directed Ru-catalyzed C(sp3)–H α-alkylation of cyclic amines with alkenes. For less-reactive ring sizes (piperidine) the acid additive is crucial to achieve full conversion of the substrate. Kinetic studies revealed that the carboxylic acid aids catalyst initiation, increases catalyst longevity, and induces a profound selectivity shift compared with the acid-free reaction, i.e., promotes alkylation over the competing alkene reduction. This is an unprecedented role of an acid in direct transition-metal-catalyzed C(sp3)–H functionalizations.
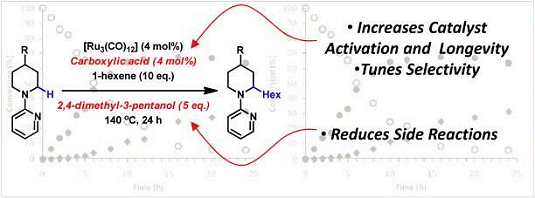
The team have utilized this protocol to effectively synthesize a number of 2-hexyl- and 2,6-dihexyl piperidines. Moreover, they have successfully synthesized the alkaloid (±)-solenopsin A by using this methodology.
Image: © Wiley-VCH
- The Role of the Alcohol and Carboxylic Acid in Directed Ruthenium-Catalyzed C(sp3)–H α-Alkylation of Cyclic Amines,
S. D. Bergman, T. E. Storr, H. Prokopcová, K. Aelvoet, G. Diels, L. Meerpoel, B. U. W. Maes,
Chem. Eur. J. 2012.
DOI: 10.1002/chem.201201072