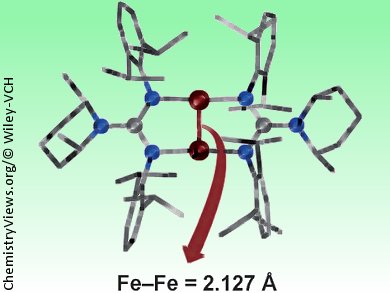
Cameron Jones and co-workers, Monash University, Australia, have synthesized an iron(I) dimer that has the shortest Fe−Fe bond (2.127 Å) reported to date. The researchers reacted a bulky guanidinato iron(II) precursor with a magnesium(I) reducing agent to produce the target iron(I) dimer. The central [Fe2]2+ unit is bridged by two delocalized guanidinate ligands and is essentially planer, this, together with the short Fe-Fe distance, suggest the presence of Fe-Fe multiple bonding.
Magnetic studies showed that the three-coordinate Fe(I) centers are high-spin, which, in the opinion of an independent referee, is an unprecedented example. The corresponding manganese(I) dimer was also prepared, and was shown to possess an unsupported carbonyl-free Mn−Mn single bond.
- Low-Coordinate Iron(I) and Manganese(I) Dimers: Kinetic Stabilization of an Exceptionally Short Fe−Fe Multiple Bond,
Lea Fohlmeister, Shengsi Liu, Christian Schulten, Boujemaa Moubaraki, Andreas Stasch, John D. Cashion, Keith S. Murray, Laura Gagliardi, Cameron Jones,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201203711 - Angew. Chem. 2012.
DOI: 10.1002/ange.201203711
Find more records in the growing list of world records in chemistry in The Chemical Record and featured on ChemistryViews.