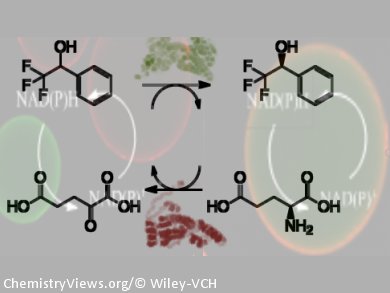
In the highly selective enzymatic synthesis of complex molecules, the in situ regeneration of the enzyme cofactor greatly improves efficiency. Less cofactor is required, unwanted products and intermediates are avoided, and thermodynamic limitations are minimized. In bi-enzymatic cascades, in which the main and recycling enzymes are co-immobilized on the same support, reaction kinetics are also improved as the recycling step takes place in situ in the porous matrix, circumventing diffusion limitations.
Fernando López-Gallego, José M. Guisan, and co-workers, Universidad Autónoma de Madrid, Spain, have co-immobilized main and recycling hydrogenases on activated agarose-type matrixes for bio-redox cascades. These systems were all more active than if the two dehydrogenases were immobilized on separate supports. For example, one system (main/recycling dehydrogenase ratio 1:5) recycled NADH up to 9000 times per substrate equivalent in 1 h at 55 °C.
The co-immobilization chemistry was optimized for each system. For example, a bio-redox cascade that used glycerol dehydrogenase and formate dehydrogenase as the NADPH recycling partner was co-immobilized reversibly by ionic exchange, whereas alcohol hydrogenase with the NADH recycling partner glutamate dehydrogenase was active if covalently co-immobilized. Performance was optimized further (1.5-fold) by a uniform distribution of enzyme and recycling partner.
- Rational Co-Immobilization of Bi-Enzyme Cascades on Porous Supports and their Applications in Bio-Redox Reactions with In Situ Recycling of Soluble Cofactors,
Javier Rocha-Martín, Blanca de las Rivas, Rosario Muñoz, José M Guisán, Fernando López-Gallego,
ChemCatChem 2012, 4, 1279–1288.
DOI: 10.1002/cctc.201200146