Organic chemists applied modern synthetic methods to the total synthesis of strychnine, which was first carried out by Nobel Laureate Robert Woodward in 1954. Get some practice in synthetic organic chemistry by deducing their route!
Before getting started with the total synthesis of strychnine, you might consider the general approach of the authors.
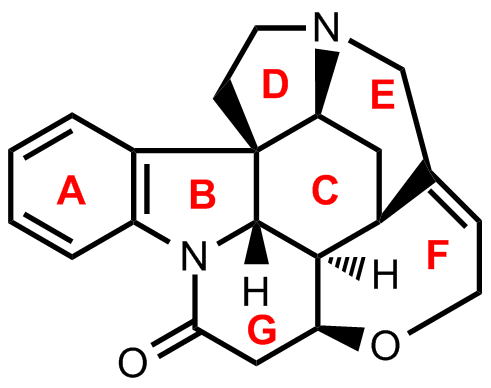
Strychnine is an alkaloid composed of seven fused rings, A – G.
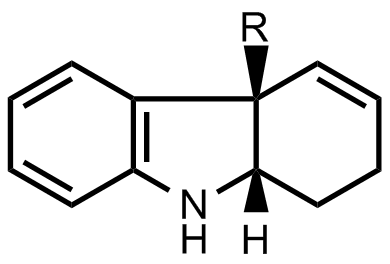
- The researchers found a way to prepare enantiomerically pure indoline skeletons. They used this method to prepare the first important intermediate.
- The scientists decided to synthesize the following tetracyclic ketone next.
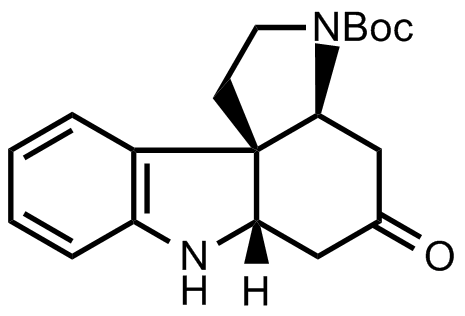
Consider the protecting groups. This will help you later to figure out protection and deprotection steps. - Then follows the synthesis of ring G of strychnine. On paper, there are two reactive sites in the tetracyclic ketone that give two possible synthetic approaches. You should figure out both possibilities as only one of them might work out. By considering the blanks in the scheme below, it will be easy to decide which one leads to experimental success.
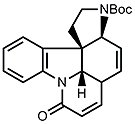
- A chemically similar route was chosen for the synthesis of ring E. After deprotection of the silyl ether (R = OH), this yields isostrychnine.
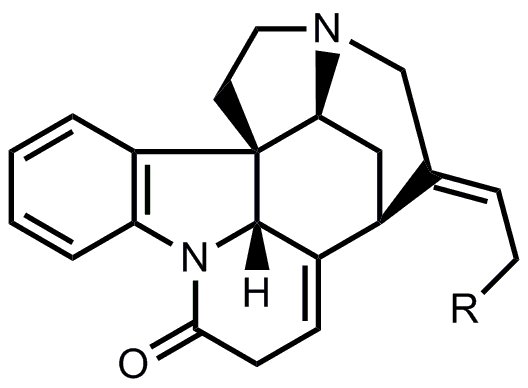
- The last cyclization from isostrychnine to strychnine corresponds to the one from Woodward’s famous synthesis of strychnine.
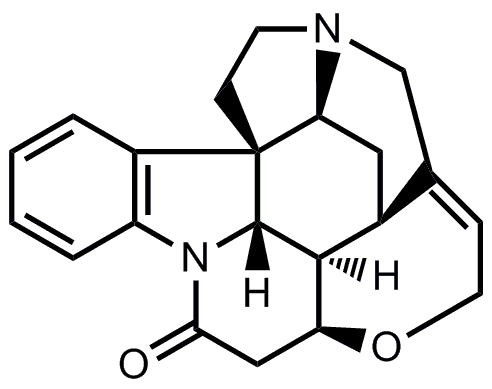
Now, try to fill in the blanks.
.png)
Hints
- To find the correct reaction conditions, you should consider the authors’ wish to emphasize the importance of palladium catalysis in natural product synthesis. This hint is also valid for the rest of the exercise.
- If you manage to work out this one, you are a truly skilled organic chemist. A one-step transformation did the job in our “age of synthetically useful palladium”.
- You have lots of options here. In this case, however, you should pick a very common route and stay away from any metals.
- Consider point 3 of the general approach. Remember that protection and deprotection steps go into individual blanks.
- Consider the position of the double bond.
- If you cannot figure out what LiAlH4 is supposed to do – it effects an unusual structure change, indeed – take into account that ring G is closed by relying on Pd catalysis once more – and that no reductant participates in this step. How many C=C bonds are there in the hexacycle once it is formed?
The solution and the publication where this synthesis was taken from will be published on January 2, 2013.
Or you may request it earlier via e-mail from [email protected]; just place “OC Quiz 1” in the subject heading.
Good Luck!
The solution can be found here.