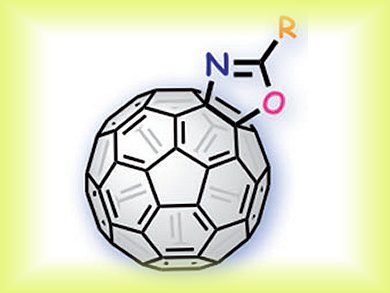
Chemical functionalization of fullerenes offers great opportunities for the creation of novel nanocarbon-based materials. Fullerooxazoles are fullerene derivatives fused with an oxazoline framework at a [6,6]-junction. Conventional synthetic methods for fullerooxazoles are indirect approaches, which require the use of highly toxic and explosive azides, as well as high temperatures for the rearrangement.
Satoshi Minakata and colleagues, Osaka University, Japan, synthesized fullerooxazoles from C60 and readily available carboxamides in a straightforward and versatile way. Their method uses radical pathways under mild reaction conditions at room temperature with a high tolerance of functional groups.
Systematic investigation of the properties, such as solubility, thermostability, and electrochemical behavior, show that control of the frontier orbital energies would be difficult to achieve by introducing functional groups onto the oxazoline ring. The method allows to regulate the solubility and thermostability of the fullerene derivatives by installing proper substituents.
- Straightforward and Versatile Synthesis of Fullerooxazoles from C60 and Carboxamides through Radical Reactions under Mild Conditions,
Youhei Takeda, Satoru Enokijima, Toshiki Nagamachi, Kazuhisa Nakayama, Satoshi Minakata,
Asian J. Org. Chem. 2012.
DOI: 10.1002/ajoc.201200114