New Fingerprinting Method Specifically for Paper
Paper is one of the surfaces most commonly tested for fingerprints in forensics. Unfortunately, it is particularly difficult to make fingerprints on paper visible. In the journal Angewandte Chemie, Israeli scientists have now introduced a new method developed specifically for use on paper. It produces a “negative” of the fingerprint and is, in contrast to conventional methods, independent of the composition of the sweat residue left behind.
In many criminal cases, paper evidence plays an important role and it would be useful to know through whose hands checks, documents, or paper currency have passed. Studies have shown that only about half of the fingerprints present on paper can be made sufficiently visible. The main reason that this does not work consistently seems to be the highly variable composition of the sweat left behind on the paper.
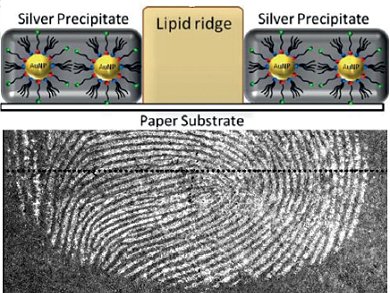
A team led by Daniel Mandler and Joseph Almog at the Hebrew University of Jerusalem has now developed a procedure that avoids these problems. It involves a sort of inversion of an established method in which gold nanoparticles are first deposited onto the invisible fingerprints, followed by elemental silver, similar to the development of a black and white photograph. In the conventional technique, the gold particles get stuck to components of the sweat in fingerprints. In contrast, the gold nanoparticles in the new method stick directly to the paper, not the sweat. This technique uses the sebum from the fingerprints, which effectively shields the paper beneath it from the gold nanoparticles. Treatment with a developer containing silver, which turns the areas with gold on them black, results in a negative image of the fingerprint.
Bifunctional Reagent that Binds to Paper Not Sweat
The secret to the success of these researchers is a special bifunctional reagent. The head of this molecule is an acylpyridazine group, which can bind to cellulose. The tail is made of hydrocarbon chains with a sulfur-containing group at the end, which binds to gold and attaches the molecule to the surface of the gold nanoparticles. When gold particles coated with these molecules are deposited onto paper with a fingerprint on it, the heads bind to the cellulose in the paper, avoiding the fat-containing lines.
Because only the fatty components of the fingerprints are used, the possibly unfavorable composition of the sweat in the fingerprint plays no role in this method. This technique also promises to alleviate another problem: if paper has become wet, it has previously been nearly impossible to detect fingerprints because the amino acids in the sweat, which are the primary substrate for current chemical enhancement reactions, are dissolved and washed away by water. The fatty components are barely effected.
- Visualization of Latent Fingermarks by Using Nanotechnology for Reversed Development on Paper: A Remedy to the Variation in Sweat Composition,
Nimer Jaber, Adam Lesniewski, Hadar Gabizon, Sanaa Shenawi, Daniel Mandler, Joseph Almog,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201205259