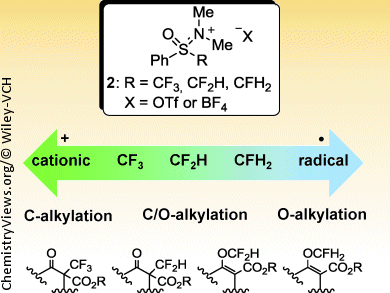
Fluorination of compounds is an important method in medicinal chemistry and is preferably performed at a late stage during synthesis of molecules. The C/O-regioselectivity in enolate alkylation is dependent on factors including the carbonyl compound, alkylating reagent, and reaction conditions. S. Tsuzuki, National Institute of Advanced Industrial Science and Technology, Japan, and N. Shibata and coworkers, Nagoya Institute of Technology, Japan, describe a unique observation regarding C/O-regioselective alkylations of β-ketoesters using fluorinated methylsulfoxonium salts 2.
Whereas trifluoromethylation selectively takes place at the C atom, monofluoromethylation of the same β-ketoester selectively occurs at the O atom. A corresponding difluoromethylation agent gave a mixture of C- and O-alkylated products. Detailed experimental observations show that C/O regioselectivity is independent of the carbonyl substrate, base or solvent, but highly dependent on the number of fluorine atoms in the fluoromethyl agent. Computational results strengthen the hypothesis that tri- and monofluoromethylations involve the formation of a more cationic +CF3 or a more radical •CFH2 species, respectively.
 Cation versus Radical: Studies on the C/O Regioselectivity in Electrophilic Tri-, Di- and Monofluoromethylations of β-Ketoesters,
Cation versus Radical: Studies on the C/O Regioselectivity in Electrophilic Tri-, Di- and Monofluoromethylations of β-Ketoesters,
Yu-Dong Yang, Xu Lu, Guokai Liu, Etsuko Tokunaga, Seiji Tsuzuki, Norio Shibata,
ChemistryOpen 2012, 1(5), 221–226.
DOI: 10.1002/open.201200032
ChemistryOpen – the first society-owned, open-access, chemistry journal – is a journal of ChemPubSoc Europe published by Wiley-VCH.