Mercury is a highly toxic heavy metal; mercury contamination comes from a variety of sources, both natural (e.g., volcanic activity) and industrial (e.g., mining, fossil fuels). Ionic mercury is easily converted into the neurotoxic monomethylmercuric cation (methylmercury) by microorganisms, which then builds up in aquatic organisms and subsequently accumulates in higher organisms through the food chain. Mercury exposure can cause serious damage to the brain, kidneys, and nervous system.
Thus, it is important to monitor the level of mercury in water and develop a simple, yet environmentally friendly, mercury sensor with high selectivity and sensitivity. Optical sensors represent the best solution for on-site environmental detection thanks to their low-cost instrumentation, fast data collection, and no need for sample pretreatment.
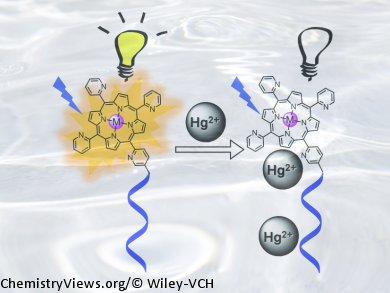
Milan Balaz and co-workers, University of Wyoming, USA, have found that zinc(II) porphyrin–octadeoxythymidine is a highly sensitive and selective fluorescence and absorption sensor for HgII ions in water. The spectroscopic detection relied on HgII-induced changes in the porphyrin–DNA and porphyrin–porphyrin interactions, resulting in quenching of porphyrin emission and redshift of the porphyrin Soret band absorption. The mercury sensor provided an emission limit of detection of 21.14 nmol L–1.
A variety of other cations (Zn2+, Cd2+, Pb2+, Mn2+, Ca2+, Ni2+, Mg2+, Cu2+, Fe2+, and Na+) did not interfere with the HgII sensing by UV/Vis absorption and emission spectroscopies, providing excellent selectivity for detection of HgII.
- Highly Sensitive and Selective Spectroscopic Detection of Mercury(II) in Water by Using Pyridylporphyrin–DNA Conjugates,
Jung Kyu Choi, Gevorg Sargsyan, Amanda M. Olive, Milan Balaz,
Chem. Eur. J. 2012.
DOI: 10.1002/chem.201202461




congratulations…..