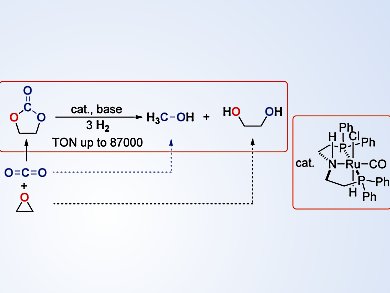
Researchers at the Shanghai Institute of Organic Chemistry, China, have shown how a readily available pincer-type (PNP)RuII catalyst can be used to produce methanol and the corresponding diols in the homogeneous hydrogenation reaction of cyclic carbonates, which are prepared from CO2 and epoxides. The reaction proceeds under mild conditions, and can result in an 89 % conversion of ethylene carbonate to form methanol (84 %) and ethylene glycol (87 %) in 72 hours, even with a catalyst loading of 0.0001 mol %.
Kuiling Ding and his team investigated the reaction of a range of different substrates, including optically active carbonates, and also by using D2. They postulate a mechanism that involves elimination of HCl from the RuII complex, followed by heterolytic cleavage of H2, and nucleophilic addition of the resulting Ru dihydride to the carbonyl group of the carbonate molecule. The NH group in the PNP ligand also plays an important role in assisting the reduction of the carbonyl group.
This procedure represents a new link between the reaction of carbon dioxide and epoxides to form carbonates, and the reaction of carbonates to produce alcohols. The team also points out that this process has potential in the utilization of waste poly(propylene carbonate) and the preparation of deuterated methanol.
- Catalytic Hydrogenation of Cyclic Carbonates: A Practical Approach from CO2 and Epoxides to Methanol and Diols,
Z. Han, L. Rong, J. Wu, L. Zhang, Z. Wang, K. Ding,
Angew. Chem. 2012.
DOI: 10.1002/ange.201207781 - Catalytic Hydrogenation of Cyclic Carbonates: A Practical Approach from CO2 and Epoxides to Methanol and Diols,
Z. Han, L. Rong, J. Wu, L. Zhang, Z. Wang, K. Ding,
Angew. Chem. Int. Ed. 2012.
DOI: 10.1002/anie.201207781