Routes to countless molecules, natural products or otherwise, with one or more cyclic moieties have contained a ring-closing metathesis (RCM) reaction that generates a small-, medium-, or large-ring olefin.
The first class of catalytic olefin metathesis reactions that provide high efficiency and Z selectivity for the synthesis of macrocyclic disubstituted alkenes has been developed. Transformations are promoted by catalysts developed by A. H. Hoveyda, R. R. Schrock and colleagues at Boston College and Massachusetts Institute of Technology, both USA, who performed the research in collaboration with D. J. Dixon and co-workers at the University of Oxford, UK.
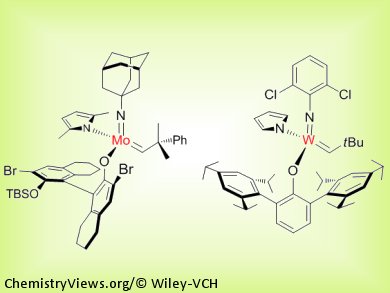
A variety of large-ring structures can be prepared stereoselectively through transformations promoted by Mo- or W-based monoaryloxide pyrrolide catalysts. Unprecedented levels of efficiency and stereoselectivity are delivered, and yields are near-doubled in cases where previous catalytic systems afforded a mixture of typically inseparable olefin isomers. These catalytic Z-selective cyclizations can be performed efficiently on gram scale with complex-molecule starting materials and catalysts that can be handled in air. Utility of the reaction is illustrated through the total syntheses of five natural products, including the anticancer and antibacterial agents epothilones A and C and nakadomarin A.
- Efficient and Selective Formation of Macrocyclic Disubstituted Z Alkenes by Ring-Closing Metathesis (RCM) Reactions Catalyzed by Mo- or W-Based Monoaryloxide Pyrrolide (MAP) Complexes: Applications to Total Syntheses of Epilachnene, Yuzu Lactone, Ambrettolide, Epothilone C, and Nakadomarin A,
C. Wang, M. Yu, A. F. Kyle, P. Jakubec, D. J. Dixon, R. R. Schrock, A. H. Hoveyda,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201204045