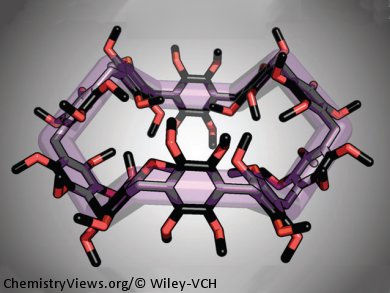
A simple one-step reaction has opened the way to a new family of large aromatic macrocycles. By simply reacting asarol methyl ether, a component of ginger, with paraformaldehyle in the presence of BF3•OEt2, Fraser Stoddart and his group at Northwestern University, Evanston, USA, were able to prepare a whole new family of macrocycles, named asararenes, in a single step. Single-crystal X-ray crystallography revealed the solid-state structures for asar[n]arenes with n = 6–11, highlighting the different structural features of these macrocycles. Even larger macrocyles with up to 15 asarol methyl ether units were detected by high-resolution mass spectrometry.
Potential applications of these materials include gas storage, building blocks for mechanically interlocked molecules, and as molecular receptors. Just as cyclodextrins and curcurbiturils have become household names in supramolecular chemistry, materials science, and nanotechnology, Fraser Stoddart envisages that asararenes could also become essential members of the molecular toolbox in the fullness of time.
- Asararenes—A Family of Large Aromatic Macrocycles,
S. T. Schneebeli, C. Cheng, K. J. Hartlieb, N. L. Strutt, A. A. Sarjeant, C. L. Stern, J. F. Stoddart
Chem. Eur. J. 2013, 19.
DOI: 10.1002/chem.201204097




Can we liquefied hydrogen storage without refrigeration or pressure ?