Making Emulsions
A new process for generating nanometer-scale oil droplets in water has been reported in the journal Angewandte Chemie by Japanese researchers, who have developed a technique they named MAGIQ (monodisperse nanodroplet generation in quenched hydrothermal solution). Under standard conditions, hydrocarbons and water do not mix; however, at high temperatures and high pressures near the critical point of water, they freely mix. Quenching homogeneous solutions of dodecane and water under these conditions in the presence of a detergent produces nanoemulsions in just ten seconds.
Oil and water are not miscible but can form emulsions in which tiny droplets of one component are dispersed in the other. Milk, face creams, and printer’s ink are examples of emulsions. Nanoemulsions with droplets that have diameters in the 20 to 200 nm range have recently attracted more attention. Because of their small droplet size, they are transparent or translucent and are much slower to separate. In addition, there are potentially interesting new applications for them, either in pharmaceutical or cosmetic formulations that are easier to absorb, or as “nanoreactors” for the production of nanomaterials.
Homogenous Solution of Oil and Water
Emulsions are usually made by a “top-down” process. Mixtures of water, oil, and surfactant are subjected to external forces, such as vigorous stirring, to break up larger drops into smaller ones. This becomes harder as the droplets get smaller, so this method has inherent limits. In contrast, solid nanoparticles are usually produced in a “bottom-up” process. This begins with a homogeneous solution. The dissolved molecules aggregate to make nanoparticles. This could also be a possible method to make nanodroplets. The problem is that water and oil would have to form a homogeneous solution to start from, but they are not miscible.
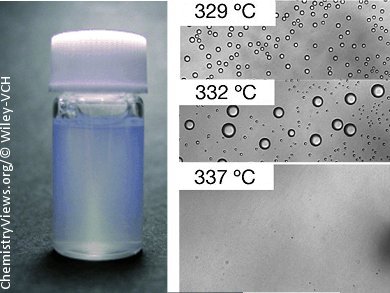
Shigeru Deguchi and Nao Ifuku at the Japan Agency for Marine-Earth Science and Technology in Yokosuka have now found a way around this with their new MAGIQ process. When water is heated under pressure it reaches its critical point at 374 °C and 22.1 MPa. At this point there is no longer a difference between the liquid and gas phases. The water no longer dissociates and no clusters of water molecules can form. At this point, the properties of the water are like those of an oil—the researchers used dodecane in this case—and the two can be freely mixed together. When this homogeneous solution is quenched with cold water, a very rapid phase separation occurs, resulting in extremely small droplets in less than ten seconds. Addition of a detergent stabilizes the nanoemulsion. The researchers developed an apparatus in which they can carry out their “magic” technique in a constant flow process. The cooling temperature and speed, the ratio of water to dodecane in the mixture, and the concentration of detergent determine the—very uniform—size of the droplets.
- Bottom-Up Formation of Dodecane-in-Water Nanoemulsions from Hydrothermal Homogeneous Solutions,
Shigeru Deguchi, Nao Ifuku,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201301403