Unusual antibiotic from a marine actinomycete is effective against anthrax
A new potential drug from a marine microorganism is effective against anthrax and various other Gram-positive bacteria, as reported by American scientists in the journal Angewandte Chemie. A chlorinated analogue kills off Gram-negative bacteria.
Anthrax is a dangerous infectious disease caused by the spore-forming bacterium Bacillus anthracis and transmitted by infected farm animals. For several years now, anthrax has also been feared as a biological weapon. Attacks with spore-containing letters caused five deaths in 2001.
Infection with anthrax usually requires tedious treatment with various antibiotics. Infections caught through the respiratory system are especially dangerous, often requiring continuous intravenous antibiotics. The search for effective antibiotics is thus correspondingly important.
Researchers working with William Fenical have now isolated a species of Streptomyces from near-shore sediments near Santa Barbara, California, USA. The culture extracts demonstrate significant activity against anthrax. The team from the University of California, San Diego, and Trius Therapeutics, San Diego, both USA, succeeded in isolating a molecule from this extract that kills off anthrax bacteria as well as other Gram-positive bacteria like staphylococci, enterococci, and streptococci. However, it is virtually useless against Gram-negative bacteria.
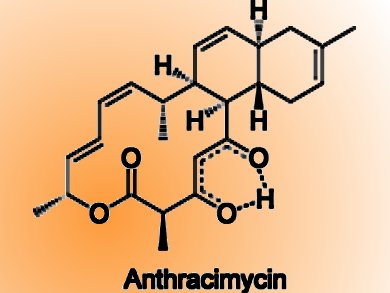
By using a variety of methods of analysis, the researchers were able to determine the structure of this molecule, which they named anthracimycin. Anthracimycin contains an unusual system of rings, one with fourteen carbon atoms and two with six each. This is a macrolide whose biosynthesis very likely occurs by the polyketide pathway. X-ray crystallographic studies allowed the researchers to determine the absolute configurations of the seven asymmetric carbon centers in this compound, identifying the complete 3-dimensional structure.
This class of molecules is completely different from all known antibiotics. An similar carbon skeleton is found in chlorotonil, a metabolite from the terrestrial myxobacterium Sorangium cellulosum. However, chlorotonil differs in its carbon skeleton, contains two chlorine atoms and the stereochemistry of most of its asymmetric carbon centers differs from that of anthracimycin.
In order to examine the effects of the chlorine atoms in the close analogue chlorotonil, the scientists chlorinated anthracimycin. This chlorine-containing analogue proved to be only about half as effective against B. anthracis. However, its activity against a number of Gram-negative pathogens increased significantly. This finding is important because Gram-negative bacteria are often resistant to current antibiotics. Comprehensive studies of this new class of antibacterials could lead to the development of effective new drugs.
- Anthracimycin, a Potent Anthrax Antibiotic from a Marine-Derived Actinomycete,
Kyoung Hwa Jang, Sang-Jip Nam, Jeffrey B. Locke, Christopher A. Kauffman, Deanna S. Beatty, Lauren A. Paul, William Fenical,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201302749