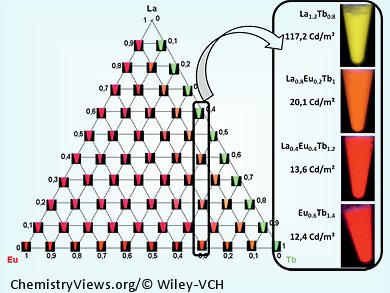
Lanthanides have intense luminescence. This can be used in applications such as screen displays and luminescent tagging. Lanthanide terephthalate coordination polymers are especially convenient for applications, as they can be synthesized very easily from aqueous solutions at room temperature with almost quantitative yield. Moreover, the terephthalate or benzene-1,4-dicarboxylate, bdc2–, ligand is nontoxic and commercially available at low cost.
Laurent Le Pollès and Olivier Guillou, Institut des Sciences Chimiques de Rennes, France, and Jean-Claude Bünzli, École Polytechnique Fédérale de Lausanne, Switzerland, and co-workers prepared a family of lanthanide terephthalate coordination polymers of the general formula [Ln2–2xLn′2x(bdc)3(H2O)4]∞, which contained the atoms La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, and Y. The team performed a very thorough study of the polymers’ colorimetric and spectroscopic properties in order to enable easy tuning of the color and brightness of the lanthanide-centered emission by adjusting intermetallic distances.
The luminescence could be excited by a wavelength that is cheaply available. Moreover, it was possible to synthesize heteronuclear compounds containing only a few percent of Eu3+ or Tb3+ ions and exhibiting luminescence almost as bright as that exhibited by the corresponding homonuclear compound. It was also possible to enhance the brightness, without modifying the color of a luminescent compound, by adding a few percent of inactive lanthanide ions. This is interesting because optically active rare earth metals are much more expensive than lanthanum.
- Color and Brightness Tuning in Heteronuclear Lanthanide Terephthalate Coordination Polymers,
Victor Haquin, Mael Etienne, Carole Daiguebonne, Stéphane Freslon, Guillaume Calvez, Kevin Bernot, Laurent Le Pollès, Sharon E. Ashbrook, Martin R. Mitchell, Jean-Claude Bünzli, Svetlana V. Eliseeva, Olivier Guillou,
Eur. J. Inorg. Chem. 2013, 20, 3464–3476.
DOI: 10.1002/ejic.201300381