Fatal Folding
Mad cow disease and its cousin Creutzfeld-Jakob disease cause fatal spongy changes in brain tissue. Today, we know that these diseases are caused by prions, proteins that are folded incorrectly. A team of German researchers have now been able to follow how the diseased proteins aggregate and “infect” healthy ones on the atomic scale. Their report appears in the journal Angewandte Chemie.
How can a disease that is caused by a protein instead of a virus or bacterium be contagious? It is clear that incorrectly folded prion proteins must be able to deform their correctly folded analogues and to change their spatial structure. They transfer their own incorrect shape to the healthy proteins. Normally, these proteins exist as monomers that are mostly wound into an alpha helix. When incorrectly folded, the protein has many regions containing beta sheets, structures that resemble an accordion, and has a tendency to self-assemble into larger aggregates. These amyloids cannot be broken down and thus form deposits in the brain’s tissue.
How this process works in detail has now been clarified. Kai Schlepckow and Harald Schwalbe, Goethe University Frankfurt am Main, Germay, have successfully used time-resolved NMR spectroscopic studies to follow what is happening to every individual amino acid as the prion protein molecules aggregate—an extremely complex process.
Two Step Aggregation
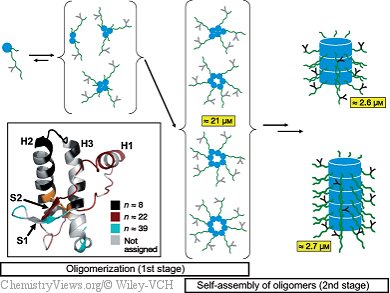
Their most interesting revelation is that the aggregation occurs in two steps. First, oligomers are formed from five to eight units. In the second step, these aggregate further into molecules made of up to 40 units that form fibrous structures. The first oligomerizations initially affect proteins in a largely unfolded state. Certain regions of the protein stiffen as the oligomerization proceeds. Different regions of the protein participate in different phases of the aggregation.
The researchers hope to use their new understanding to better determine what role is played by the specific mutations in the prion protein that seem to fuel initiation of this process. This may also provide a starting point for the development of effective drugs.
- Molecular Mechanism of Prion Protein Oligomerization at Atomic Resolution,
Kai Schlepckow, Harald Schwalbe,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201305184