Synthetic Heparin Mimetics
One of the risks of any large operation is the occurrence of blood clots. To prevent this, patients are routinely given the anticoagulant heparin or related drugs. American scientists have now introduced a new approach to the production of synthetic heparin mimetics with better activity profiles.
Heparin has been used as an anticoagulant since 1935 to both treat and prevent the deep vein thrombosis that can result from operations, blood transfusions, or dialysis. Heparin is a substance produced by the body and consists of long chains of sugar (saccharide) molecules. The sugar building blocks contain a large number of sulfate groups.
Because heparin is obtained from animal tissues, its use does pose some problems. Contamination may lead to health risks. Furthermore, batches of the drug are often not homogeneous so the effectiveness of a given dose must be calculated case by case. In about 3 % of patients, long-term treatment with heparin leads to a dangerous autoimmune disease. Low-molecular-weight drugs such as Arixtra, which contains only five sugar groups, have been developed as an alternative. Their disadvantage is the very complex and expensive process used to make them.
Fine-tuning of the Anticoagulant Effect
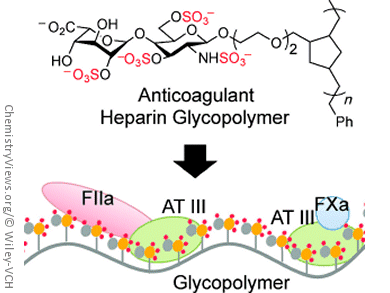
Linda C. Hsieh-Wilson and her team, California Institute of Technology, Pasadena, have now uncovered an interesting new angle: synthetic glycopolymers, long chains of molecules that have sugar molecules as side groups. The researchers chose to use two sugars typically found in heparin as side groups. One of these sugars was equipped with an additional sulfate group. The synthesis of such glycopolymers is much simpler than the synthesis of natural polysaccharides, but it is still a complex undertaking, and it is made more difficult in this case because of the need to attach sulfate groups in a controlled fashion. The team was able to use a ring-opening metathesis polymerization reaction (ROMP) to make polymer chains of varying length with a maximum of 45 units.
The longer molecular chains demonstrate stronger activity than anticoagulants currently in clinical use. The additional sulfate group is critical to this effectiveness. Interestingly, systematic changes to the length of the chain and pattern of sulfate groups allow for fine-tuning of the anticoagulant effect. This makes it possible to make drugs with different activities from those previously in clinical use. For example, the glycopolymer containing 45 building blocks targeted the two major branches of the blood coagulation cascade to a different extent than both the small molecule and heparin polysaccharide drugs.
- Tailored Glycopolymers as Anticoagulant Heparin Mimetics,
Young In Oh, Gloria J. Sheng, Shuh-Kuen Chang, Linda C. Hsieh-Wilson,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201306968