β-Hydroxy ketones are useful compounds in organic synthesis, and facile C–C bond cleavage in these compounds by the retroaldol reaction has also been applied in the synthesis of complex molecules. 3-Hydroxycyclobutanones are isolable, four-membered, small-ring β-hydroxy ketones. Their structural features have potential for developing unique synthetic reactions.
An interesting reaction of 3-hydroxybutanones has been unveiled by Jun-ichi Matsuo and co-workers, Kanazawa University, Japan. They found that activation of 3-hydroxycyclobutanone with boron trifluoride etherate in the presence of α,β-unsaturated ketones provides cyclohexanone derivatives by formal [4+2] cycloaddition at C–C double bond of the α,β-unsaturated ketones. A 3-hydroxy group on the cyclobutanone ring is essential for the chemoselectivity towards the C–C double bond, as similar treatment of 3-ethoxycylobutanones gives dihydro-γ-pyrones by the reaction at C–O double bond of α,β-unsaturated ketones (see scheme below).
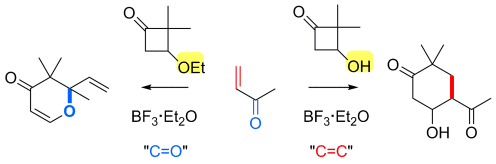
Intentional control of chemoselectivity of cycloaddition is usually difficult, but this method makes it possible by proper protection of the 3-hydroxy group of cyclobutanones.
- Chemoselective Formal [4+2] Cycloaddition of 3-Hydroxycyclobutanones with Enones,
Kosuke Harada, Aya Nowaki, Jun-ichi Matsuo,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300156
![[4+2] = Cyclohexanones](https://www.chemistryviews.org/wp-content/uploads/legacy/common/images/thumbnails/source/1420f3c3702.gif)



