Hydrazones are potentially valuable reagents for organic synthesis. The conventional method for the preparation of hydrazones involves mixing a carbonyl compound, such as an aldehyde and a ketone, with a hydrazine.
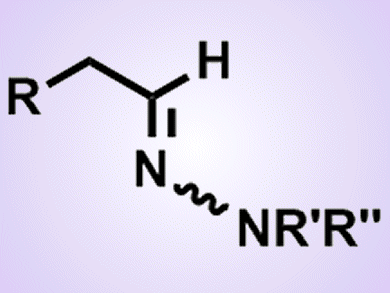
The addition of hydrazines to alkynes, namely hydrohydrazination, is an alternative and atom-economical route to hydrazones. In most cases, hydrohydrazination of terminal alkynes proceeds in a Markovnikov manner to give methyl ketone hydrazones.
Yoshiya Fukumoto and co-workers, Osaka University, Japan, have developed the anti-Markovnikov addition of hydrazines to terminal alkynes in the presence of [TpRh(C2H4)2]/P(2-fulyl)3 catalyst (Tp = trispyrazolylborate). Both Tp and P(2-fulyl)3 are crucial ligands for the regioselective hydrohydrazination. Various N-alkyl and N,N-dialkylhydrazines can undergo the reaction to afford the corresponding aldimine-type hydrazones.
Further research focuses on further applications of the hydrazone products as well as studying the reaction mechanism in depth.
- Rhodium-Catalyzed Anti-Markovnikov Hydrohydrazination of Terminal Alkynes with N-Alkyl- and N,N-Dialkylhydrazines,
Yoshiya Fukumoto, Akihiro Ohmae, Masaya Hirano, Naoto Chatani,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300188