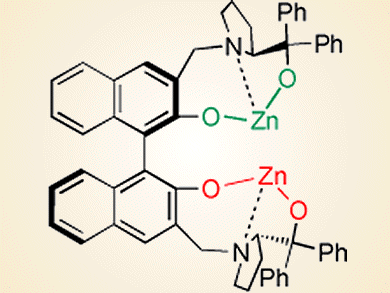
Bifunctional homobimetallic catalysts are attractive for use in organic reactions. C2-symmetry is a generally applied feature of the skeleton in the design of chiral catalysts and chiral zinc compounds are among the most successful catalysts in a variety of asymmetric reactions. Catalytic asymmetric hydrophosphonylation of aldehydes is considerably important in producing biologically active compounds. Neither homobimetallic zinc nor organozinc compounds have been reported for catalyzing asymmetric hydrophosphonylation, although several successful protocols with other catalysts have been disclosed and give high enantioselectivity.
Chao-Shan Da and co-workers, Lanzhou University, China, have reported a C2-symmetric homobimetallic Zn/BINOL-based catalyst (see picture) for the asymmetric hydrophosphonylation of aldehydes. It is interesting that the two identical zinc alkoxides of the catalyst serve two distinct roles. One acts as a Brønsted base and activates the dialkyl phosphonite, and the other acts as a Lewis acid to activate the aldehyde.
This work expands the asymmetric versions of the hydrophosphonylation and offers an interesting insight into future bifunctional catalyst designs.
- C2-Symmetric Homobimetallic Zinc Complexes as Chiral Catalysts for the Highly Enantioselective Hydrophosphonylation of Aldehydes,
Lin Sun, Qi-Peng Guo, Xiao Li, Lei Zhang, Yu-Yan Li, Chao-Shan Da,
Asian J. Org. Chem. 2013.
DOI: 10.1002/ajoc.201300181