Fe3O4-based nanostructured materials are attracting attention in the search for new high-capacity anode materials for the next generation of lithium-ion batteries (LIBs). This is due to their high theoretical capacity of approximately 928 mA h g–1, which is about three times that of conventional graphite, as well as their low cost, low toxicity, eco-friendliness, and the natural abundance of iron. However, poor cyclic performance, rate capability, and mechanical degradation resulting in hastened loss of capacity has hindered their practical application. Two adaptations have been applied to mitigate these issues. Firstly, use of carbon coatings and secondly, the fabrication of hollow and/or porous nanostructures. However, these two strategies have only given limited improvement in electrochemical performance.
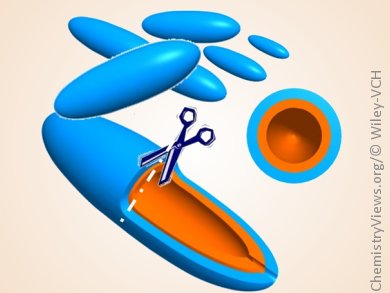
An-Hui Lu, Dalian University of Technology, China, and colleagues have combined the two approaches and synthesized a single Fe3O4-based hybrid material with a hollow/penetrated mesochannel structure and carbon coating in the hope that it will significantly improve the electrochemical performance. The synthesis, termed “confined nanospace pyrolysis”, involved the production of a polydopamine coating followed by a silica coating on a rice-grain-shaped β-FeOOH nanoparticle, and subsequent treatment by using confined nanospace-pyrolysis and silica-removal procedures. The resultant coaxial hollow Fe3O4@C (pictured) possessed a rice-grain morphology and mesoporous structure with a large specific surface area, as well as a continuous and flexible carbon shell.
Electrochemical tests showed Fe3O4@C to have a high specific capacity (ca. 864 mA h g–1 at 1 A g–1), excellent rate capability with a capacity of about 582 mA h g–1 at 2 A g–1, and a high Coulombic efficiency (>97 %). The hollow cavity and flexible carbon shell allow the particles to accommodate the volume change of the Fe3O4 during the lithium insertion/extraction processes. Additionally, the coaxially penetrated mesochannel structure has small open holes at the ends of the particles, which allow electrolyte access and thus facilitate the rapid diffusion of lithium ions from the electrolyte to the active materials.
- Confined Nanospace Pyrolysis for the Fabrication of Coaxial Fe3O4@C Hollow Particles with a Penetrated Mesochannel as a Superior Anode for Li-Ion Batteries,
Cheng Lei, Fei Han, Qiang Sun, Wen-Cui Li, An-Hui Lu,
Chem. Eur. J. 2013.
DOI: 10.1002/chem.201303175