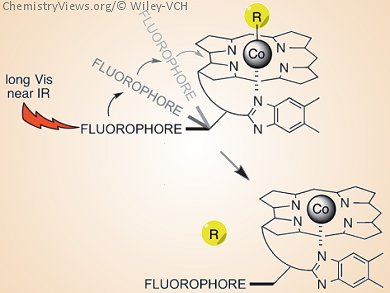
Researchers in the USA have shown how the attachment of light-capturing antennas to substituted cobalamins results in cleavage of the Co–C bond at wavelengths up to 800 nm (see picture). Thomas A. Shell, David S. Lawrence, and their team, University of North Carolina, Chapel Hill, USA, synthesized a set of wavelength-tunable photoresponsive species that can be excited in the visible and near-IR range, as excitation at wavelengths shorter than 450 nm can result in biological damage and the optical window of tissue is from 600−1000 nm.
The team attached fluorophores – including TAMRA, SulfoCy5, Alexa700, and Bodipy650 – to the cobolamin framework. Photolysis of the Co−C bond occurred when the system was excited at the major fluorophore excitation band. The range of possible excitation wavelengths means that mixtures of compounds can be photolyzed in an illumination sequence.
The team applied their principle to inter-organelle trafficking: a cobalamin-Bodipy650 conjugate (connected through the Co center) was sequestered in endosomes. After illumination, the Bodipy650 is transferred from the endosomes to the mitochondria. In a second application, illumination of a cobalamin–doxorubicin conjugate resulted in release of doxorubicin and apoptosis of HeLa cells. A third example was the use of a cobolamine−cAMP conjugate to control the activity of a protein kinase signaling pathway.
This ability to use long-wavelength visible and near-IR light to control bioactive species can be used in many areas, including drug delivery, nanotechnology, and materials fabrication.
- Tunable Visible and Near-IR Photoactivation of Light-Responsive Compounds by Using Fluorophores as Light-Capturing Antennas,
Thomas A. Shell, Jennifer R. Shell, Zachary L. Rodgers, David S. Lawrence,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201308816