Organosuperbases that have high basicity and stability as well as low nucleophilicity are extremely useful in organic synthesis. However, they can have several disadvantages, including high toxicity, and low solubility and stability. Ivo Leito, Ullrich Jahn, and co-workers in the Czech Republic, Estonia, and Croatia set out to synthesize organosuperbases that overcome these limitations.
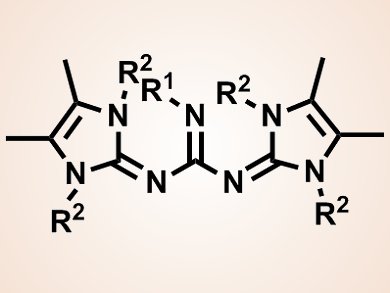
The team fused N,N’-dialkyl-4,5-dimethylimidazol-2-ylidene amine organosuperbases with a guanidine organosuperbase core to form bis-N,N’-(1,3-dialkyl-4,5-dimethyl-1H-imidazol-2(3H)-ylidene) guanidine bases (BIG bases; pictured). The pKa values of these bases in acetonitrile are between 35 and 38, which are higher than those of the constituent bases. This increased basicity results from the size of the bases, which allows more efficient delocalization of the positive charge over larger areas in the protonated form. Protonation also results in the synergistic effect of aromatization and steric strain release.
These organosuperbases are the strongest phosphorus-free bases reported to date. Their other advantages include the fact that they are easily synthesized in four steps from readily available starting materials, and that they are more stable and lipophilic than other organosuperbases, including P2-phosphazenes and guanidinophosphazenes. These bases may potentially be applied in asymmetric synthesis or organocatalysis.
- Very Strong Organosuperbases Formed by Combining Imidazole and Guanidine Bases: Synthesis, Structure, and Basicity,
Katarina Vazdar, Roman Kunetskiy, Jaan Saame, Karl Kaupmees, Ivo Leito, Ullrich Jahn,
Angew. Chem. Int. Ed. 2013.
DOI: 10.1002/anie.201307212


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
