Strong Bonds For Strong Teeth
Shark teeth are supposedly the healthiest of all animals because of their particularly hard enamel. Japanese researchers have now been able to use a special electron microscopy technique to image the structure of shark enamel. In the journal Angewandte Chemie, they report on an unusually strong bond between fluorine atoms and calcium atoms, which may be responsible for the unusual hardness and cavity resistance of shark teeth.
Biominerals play an important role for all life forms. Our own bones and teeth consist of a composite material made from biomolecules and inorganic substances. Shark tooth enamel is mainly fluorapatite (Ca5[F(PO4)3]), a mineral with a hexagonal crystal structure that contains calcium, fluorine, and phosphate. But what is it that makes the shark’s tooth enamel so particularly strong? So far, all we have determined is that it has a high density of fluorapatite crystals and a low content of organic matrix.
Imaging the Structure of Shark Enamel
Determining the exact structures of biominerals turns out to be distinctly difficult. In the best cases, experiments using transmission electron microscopy have been able to deliver information on the nanometer scale. Advances like aberration correction have improved the resolution of TEM, but the signals are weak and the structures extremely complex. In addition, the electron beam damages biominerals. A team led by Yuichi Ikuhara has now been able to examine the enamel of shark teeth by TEM and scanning TEM (STEM) with minimum interference. To achieve this, the researchers from Tohoku University, the University of Tokyo, the Graduate School of Tokyo Medical and Dental University, and the Fine Ceramics Center, all Japan, used an aberration-corrected electron microscopy technique that gets by with a very low dose. This method works by using a smaller condenser aperture and dispersing the electron beam over a wider area of the sample than usual.
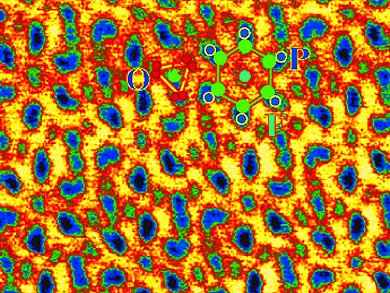
The scientists were thus able to spatially resolve each individual atom columns inside the complex fluorapatite structure. They found that shark tooth enamel consists of bundles of monocrystalline nanorods of fluorapatite with a diameter of about 50 nm. The hexagonal shape of the crystal could also be confirmed. Every hexagon consists of calcium, phosphorus, and oxygen atoms with a fluorine atom at the center. By using ab initio calculations, the researchers were able to determine that the fluorine atoms are bound to the surrounding calcium atoms with covalent–ionic mixed bonds, not ionic bonds alone as expected. This seems to be the main reason for the special cavity resistance of shark teeth.
- Fluorine in Shark Teeth: Its Direct Atomic-Resolution Imaging and Strengthening Function,
Chunlin Chen, Zhongchang Wang, Mitsuhiro Saito, Tetsuya Tohei, Yoshiro Takano, Yuichi Ikuhara,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201307689