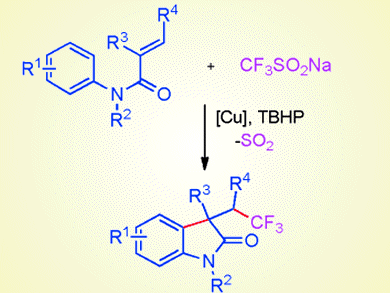
Trifluoromethyl-containing organic compounds are increasingly significant for a variety of uses, for example, as pharmaceuticals, agrochemicals, and materials. However, the direct incorporation of a CF3 group into electron-deficient hydrocarbons has remained an outstanding challenge.
Aiwen Lei and co-workers, Wuhan University, China, have developed a copper-catalyzed oxidative trifluoromethylation of electron-deficient alkenes through a radical pathway. The readily available Langlois reagent (CF3SO2Na) was used as the trifluomethylation agent. An intramolecular radical annulation process addresses the disfavored addition of an electrophilic radical to electron-poor alkenes, which plays a vital role in achieving this transformation.
A series of valuable CF3-containing oxindoles were synthesized under mild conditions with good functional group tolerance. Not only that, the group used operando IR to reveal that the generation of a CF3 radical might be a fast step.
This work provides a novel approach for efficient and selective introduction of a CF3 group into electron-deficient unsaturated organic compounds, which offers new options for further development in radical trifluoromethylation reactions.
- Copper-Catalyzed Trifluoromethylation-Initiated Radical Oxidative Annulation toward Oxindoles,
Qingquan Lu, Chao Liu, Pan Peng, Zhiliang Liu, Lijun Fu, Jinguo Huang, Aiwen Lei,
Asian J. Org. Chem. 2014, 3, 273–276.
DOI: 10.1002/ajoc.201300236