Graphene
Graphene has been considered a hot candidate for a new generation of silicon-free electronics since the discovery of this two-dimensional form of carbon. However, graphene is not a semiconductor. In the journal Angewandte Chemie, an international team of researchers has now introduced a carbon nitride, a structural analogue of graphene made of carbon and nitrogen that appears to exhibit semiconducting properties.
Graphitic Carbon Nitride
With a planar, hexagonal, honeycomb structure and freely moving electrons, graphene is, in principle, nothing more than a single-atom layer of graphite. From an electronic point of view, it is a very interesting substance – but it is missing the typical electronic band gap that would make it a semiconductor. This band gap is the difference in energy between the valence band and the conduction band of the electrons. To be effective, this gap must not be too large, so that it allows electrons to easily move from the valence band to the conduction band when excited. Various methods have previously been used to provide graphene with such a band gap. An alternative idea is to make a “graphitic carbon nitride”, a material made of carbon and nitrogen, which ought to have properties very similar to graphene. A team of researchers from the University of Liverpool, UK, the University of Ulm, Germany, the Humboldt University in Berlin, Germany, the Aalto University, Finland, University College London, UK, and the Max Planck Institute of Colloids and Interfaces in Potsdam, Germany, has now been able to make such a material for the first time.
Structure and Properties
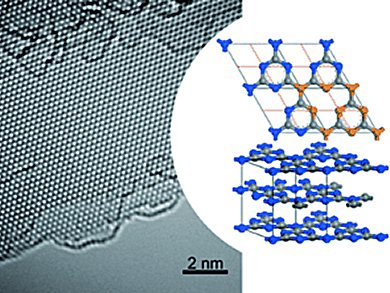
Transmission electron microscopy and scanning force microscopy, as well as X-ray crystallographic examinations proved that the thin crystalline films are a triazine-based, graphitic carbon nitride (TGCN). Triazines are six-membered rings containing three carbon and three nitrogen atoms. The new material consists of such triazine rings, with additional nitrogen atoms connecting the rings into groups of three to make a two-dimensional layer. The team led by Andrew I. Cooper and Michael J. Bojdys believes that these layers are not fully planar, but are instead slightly wavy.
TGCN thus has a structure similar to that of graphite, however—as hoped—it is a semiconductor. The films produced consisted of between three and several hundred layers of atoms with a direct band gap between 1.6 and 2.0 eV. During the production process, the layers of TGCN are preferentially deposited onto substrates. The crystallization of TGCN on the surface of insulating quartz offers potential for practically relevant applications. This may be a step on the way to the post-silicon era of electronics.
- Triazine-Based, Graphitic Carbon Nitride: a Two-Dimensional Semiconductor,
Gerardo Algara-Siller, Dr. Nikolai Severin, Samantha Y. Chong, Torbjörn Björkman, Robert G. Palgrave, Andrea Laybourn, Markus Antonietti, Yaroslav Z. Khimyak, Arkady V. Krasheninnikov, Jürgen P. Rabe, Ute Kaiser, Andrew I. Cooper, Arne Thomas, Michael J. Bojdys,
Angew. Che. Int. Ed. 2014.
DOI: 10.1002/anie.201402191