Linearly fused polyclic aromatic hydrocarbons, which are key compounds in most photonics and organic electronics applications, and their heterocyclic analogs can be easily prepared. However, this does not hold true for their vertically fused, more stable analogs. Consequently, little is known about these polycyclic heterocycles.
A team of Polish researchers reports on a journey to optimize the conditions of dehydrogenative coupling and to investigate photophysical properties of the products.
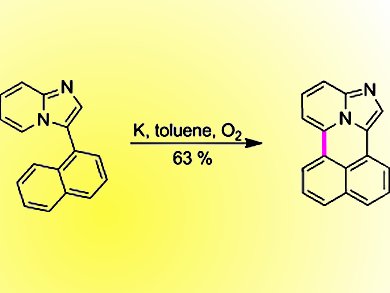
Daniel T. Gryko, Warsaw University of Technology, Poland, and Boleslaw Kozankiewicz, Polish Academy of Science, Warsaw, and their co-workers found that while oxidative aromatic coupling failed to fuse singly linked precursor into imidazo[5,1,2-de]naphtho[1,8-ab]quinolizine, anion radical coupling in the presence of dry O2 gave this novel compound in 63 % yield. This vertically fused imidazo[1,2-a]pyridine has a very low fluorescence quantum yield. This unexpected result was explained with DFT calculations and analysis in Shpol’skii matrix, which revealed that triplet excited states lie energetically close to S1, which facilitates intersystem crossing. Phosphorescence at low temperature was also observed.
- Vertically π-Expanded Imidazo[1,2-a]pyridine: The Missing Link of the Puzzle,
Dikhi Firmansyah, Marzena Banasiewicz, Irena Deperasińska, Artur Makarewicz, Boleslaw Kozankiewicz, Daniel T. Gryko,
Chem. Asian J. 2014.
DOI: 10.1002/asia.201402201