Methane is an ideal raw material for C1 chemistry due to its natural availability. The current main use of methane from an industrial point of view consists of the preparation of syngas (synthesis gas), a mixture of carbon monoxide and hydrogen that can be further employed in the synthesis of methanol, in the Fischer-Tropsch process for synthetic gasoline, or in reactions requiring carbon monoxide and/or hydrogen (hydroformylation, hydrogenation, ammonia synthesis, or carbonylation among others). Alternatively, the direct, selective, and low-energy conversion of methane into more valuable chemicals in homogeneous media still constitutes a long-term challenge.
Gregorio Asensio, Ana Caballero, Michel Etienne, Pedro J. Pérez, and co-workers, Universidad de Valencia and Universidad de Huelva, Spain, and LCC-CNRS and Université de Toulouse, both France, have developed a family of perfluorinated tris(indazolyl)borate silver complexes that are capable of catalyzing the transfer of carbene units from ethyl diazoacetate to the C–H bond of methane by using supercritical carbon dioxide (scCO2) as the reaction medium. Other light alkanes (ethane, propane, n-butane, and iso-butane) have also been functionalized and the relative intramolecular reactivity values for the C–H bonds in those alkanes have been obtained.
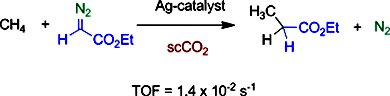
Thus, this method provides an efficient route for the catalytic functionalization of methane and light alkanes to produce more valuable synthetic products.
- Catalytic Functionalization of Methane and Light Alkanes in Supercritical Carbon Dioxide,
M. Ángeles Fuentes, Andrea Olmos, Bianca K. Muñoz, Kane Jacob, M. Elena González-Núñez, Rossella Mello, Gregorio Asensio, Ana Caballero, Michel Etienne, Pedro J. Pérez,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201403217