Chemical synthesis of oligosaccharides with multiple 1,2-cis α-glycosidic bonds is challenging.
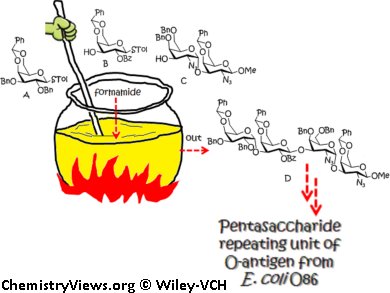
K.-K. Tony Mong and co-workers, National Chiao Tung University, Hsinchu, Taiwan, have applied a formamide-modulated glycosylation method to the synthesis of a pentasaccharide repeating unit of the O-antigen of Escherichia coli O86. Such O-antigens have been used in the past for developing vaccines. The pentasaccharide target contains three 1,2-cis α-glycosidic bonds arising from D-galactosyl, 2-acetamido-2-deoxy-D-galactosyl, and L-fucosyl residues.
Through the use of a N-formylmorpholine (NFM)-modulated glycosylation method, 1,2-cis α-glycosidic bonds from D-thiogalactosyl and 2-azido-2-deoxy-D-galactosyl donors were constructed in high selectivity without the need for specialized auxiliary functional groups. A reduction in the number of the synthetic steps was accomplished by the formamide-modulated one-pot glycosylation strategy.
- Chemical Synthesis of the O-Antigen Repeating Unit ofEscherichia coliO86 by anN-Formylmorpholine-Modulated One-Pot Glycosylation Strategy,
Arun B. Ingle, Chin-Sheng Chao, Wei-Cheng Hung, Kwok-Kong Tony Mong,
Asian J. Org. Chem. 2014.
DOI: 10.1002/ajoc.201402057



we don’t have any analyzer here to estimate the concentrations of formamide_modulated form that had been developed during the process. could you explain it more? regards Tiurma PT Simanjuntak, MSi 081370213203