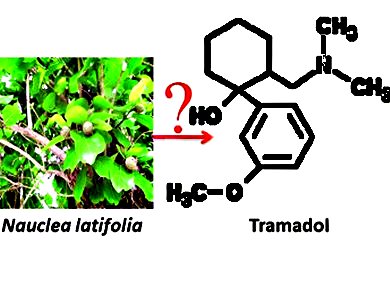
The synthetic analgesic tramadol ((±)-cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol) was recently detected by DeWaard et al. in the roots of Nauclea latifolia (Rubiaceae) [1], a plant from Cameroon that is used for medical purposes. However, the uncertainty about the biosynthetic pathway and isotope ratio analyses showing that the δ15N/14N and δ13C/12C ratios of the natural tramadol fall within the range of commercial samples, were arguments for a synthetic product. This has been discussed controversially.
Michael Spiteller, TU Dortmund, Germany, and colleagues have phytochemically analyzed the roots of N. Latifolia and other plants, as well as soil and water samples from the north and south of Cameroon at different times of the year.
They found that tramadol and its major mammalian metabolites (O-desmethyltramadol, N-desmethyltramadol, and 4-hydroxycyclohexyltramadol) are present in the roots of several plants as well as in soil and local water sources in the far north region, but not in the south.
The farmers in the north consume high doses of synthetic tramadol to work throughout the day under the sun in extremely high temperatures without getting tired. For the same reason they feed their cattle and horses with tramadol. In the south tramadol is unknown.
In vitro tests with endophytic microflora isolated from the tissues of N. latifolia strongly suggested that tramadol is not biosynthesized.
These results show that tramadol and its metabolites found in plant roots are not biosynthetic products of the plants, but a result of cross-contamination by synthetic tramadol.
Tramadol has severe side effects, so its off-label use may pose a risk to humans and animals directly dosed with tramadol, but also to those exposed indirectly by drinking water.
- Tramadol – A True Natural Product?,
Souvik Kusari, Simplice Joel N. Tatsimo, Sebastian Zühlke, Ferdinand M. Talontsi, Simeon Fogue Kouam, Michael Spiteller,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201406639
[1] A. Boumendjel, G. S. Taïwe, E. N. Bum, T. Chabrol, C. Beney, V. Sinniger, R. Haudecoeur, L. Marcourt, S. Challal, E. Ferreira Queiroz, F. Souard, M. Le Borgne, T. Lomberget, A. Depaulis, C. Lavaud, R. Robins, J.-L. Wolfender, B. Bonaz, M. De Waard, Occurrence of the Synthetic Analgesic Tramadol in an African Medicinal Plant, Angew. Chem. Int. Ed. 2013, 52 (45), 11780—11784. DOI: 10.1002/anie.201305697