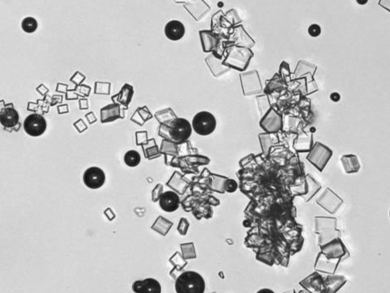
In the food industry, the crystallization of sugar is subject to various factors, for example high viscosity of sugar solutions and additives such as flavoring agents. For the development of new products, it is therefore necessary to know if impurities alter the crystallization behavior of sugar. Additives can affect various parameters of a crystallization process, for example solubility, nucleation and crystal growth rate.
Joachim Ulrich and colleagues at Martin Luther University Halle-Wittenberg, Halle, Germany, studied the influence of caffeine on the crystallization behavior of sugar solutions and found that it influences different items of crystallization. They determined parameters such as solubility, metastable zone width, nucleation, crystal growth rate, and shape of crystals. They showed that, in the presence of caffeine, the growth of the sugar crystals is slower and the solubility and nucleation temperatures are shifted. Also, the use of tap or distilled water in experiments or production plays an important role with respect to the solubility of sugar, based on the pH of the solvent.
- Influence of Caffeine on the Crystallization Behavior of Sugar
Stefanie Selbmann, Sandra Petersen, Joachim Ulrich
Chem. Eng. Technol. 2014, 37 (8), 1413 -1416.
DOI: 10.1002/ceat.201300829