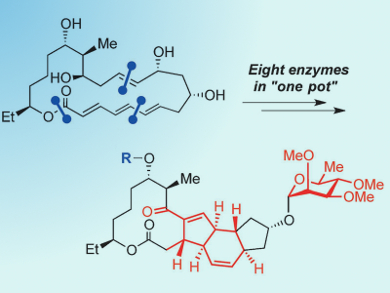
Polyketide backbones are formed by biosynthesis catalyzed by polyketide synthases (PKSs). However, post-PKS modifications result in a high degree of structural complexity that makes the products difficult to synthesize chemically. The polyketide-derived insecticide spinosyn A is an example of such a compound. Its complex forty-carbon framework comprises four fused rings and seventeen stereogenic centers. Its total synthesis has already been reported by three research groups who used different chemical routes to access the target molecule.
Hung-wen Liu and colleagues, University of Texas, Austin, USA, wanted to try a different approach: they prepared a cyclic precursor by using organic synthetic methods, and then used eight enzymatic reactions in one pot to complete the synthesis. The researchers synthesized the cyclic precursor from three fragments by Stille coupling, Julia–Kocienski olefination, and Yamaguchi macrolactonization. They subsequently reacted the precursor with the enzymes SpnF, SpnG, SpnH, SpnI, SpnJ, SpnK, SpnL, and SpnM in one pot. This reaction produced a 17-pseudoaglycone in 19.6 % yield.
One drawback was that the final forosamylation could not be carried out with SpnP and was carried out chemically. The researchers acknowledge that their approach still has a long way to go before it can be generally applied, and that has some disadvantages compared to chemical synthesis, including the requirement of a thorough understanding of the biosynthetic pathways involved. However, it does represent a promising alternative method for the synthesis of complex polyketides.
- Chemoenzymatic Synthesis of Spinosyn A
Hak Joong Kim, Sei-hyun Choi, Byung-sun Jeon, Namho Kim, Rongson Pongdee, Qingquan Wu, Hung-wen Liu
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201407806