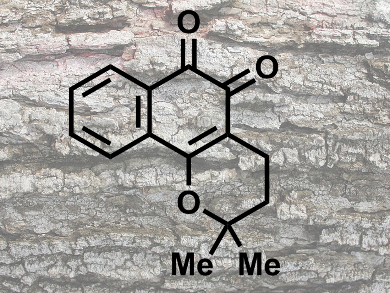
The biologically active naphthoquinones lapachol, α-lapachone, and β-lapachone (pictured) were originally isolated from the bark of the lapacho tree (Tabebuia sp.). These natural products show a wide range of biological activities such as antitumor, antibacterial, antiplasmodial, antiangiogenic, trypanocidal, and anti-inflammatory responses.
Tadashi Katoh and colleagues, Tohoku Pharmaceutical University, Sendai, Japan, have synthesized β-lapachone in 70 % overall yield in four steps. The synthesis starts from commercially available 1,4-naphthoquinone. The key step of the synthesis is a cyclization/hydration/oxidation cascade reaction of 2-prenyl-1,4-naphthoquinone leading to target β-lapachone in one step. Advantages to previously reported methods are a higher overall yield, easier operation, and a less expensive starting material.
- Synthesis of β-Lapachone, a Potential Anticancer Agent from the Lapacho Tree,
Takeru Katoh, Hiromitsu Monma, Jun Wakasugi, Koichi Narita, Tadashi Katoh,
Eur. J. Org. Chem. 2014.
DOI: 10.1002/ejoc.201403064


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
