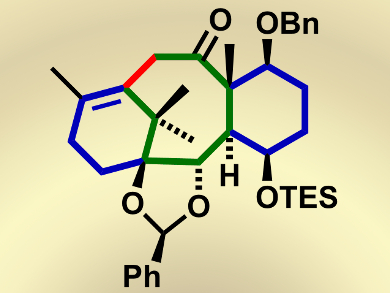
Taxol, isolated from the bark of the Pacific yew tree (Taxus brevifolia), displays potent bioactivity and has been widely used as an anticancer drug. The complex structure of taxol, which includes a highly distorted 6-8-6 taxane scaffold with nine stereogenic centers (including an all-carbon quaternary stereogenic center), diverse functional groups such as oxetane, trans-1,2-diol, acyloin, and a bridgehead double bond, combined with its biological properties has made it an attractive and challenging synthetic target.
The formation of the eight-membered carbocyclic ring, in particular, has been a significant problem in some previous syntheses because of its highly strained nature arising from transannular strain.
Masahisa Nakada and colleagues, Waseda University, Japan, have tackled these challenges by envisioning a convergent total synthesis of (–)-taxol. They prepare the initial building blocks of (–)-taxol in a parallel fashion, thus reducing time and also providing the advantage of the independent transformations of functional groups that are otherwise incompatible with each other. The problem of forming the eight-membered carbocyclic ring was then solved by using the palladium-catalyzed intramolecular alkenylation of a methyl ketone, thus affording the cyclized product in an excellent yield (96 %).
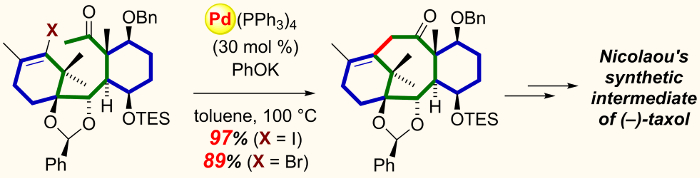
The nature of this convergent approach has the potential to enable the synthesis of diverse taxol derivatives and work is currently underway to reduce the number of synthetic steps.
- Formal Total Synthesis of (−)-Taxol through Pd-Catalyzed Eight-Membered Carbocyclic Ring Formation,
Sho Hirai, Masayuki Utsugi, Mitsuhiro Iwamoto, Masahisa Nakada,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201404295