Stable silylenes exhibit a number of intriguing and versatile reaction patterns. In particular, the reactivity of silylenes with unsaturated organic molecules has attracted a lot of attention because they provide access to small silacycles. Reactions of silylenes with alkynes predominantly yield [2+1] cycloaddition products, silacyclopropenes. Modification of silylenes by the incorporation of further reactive sites provides an opportunity for access to more diverse products thanks to their versatile reactivity.
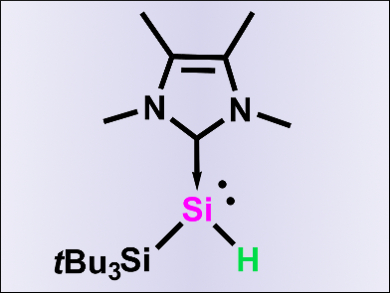
Shigeyoshi Inoue and colleagues, Technische Universität Berlin, Germany, have conducted an in-depth study into the reactivity of an N-heterocyclic carbene (NHC)-stabilized silylene monohydride, tBu3Si(H)Si–NHC, with alkynes. This compound features three reactive sites: the lone pair on silicon, the Si–H bond, and the NHC.
Reaction of the silyene monohydride with phenylacetylene yielded supersilyl-substituted 1-alkenyl-1-alkynylsilane, tBu3Si(H)Si(CHC=CHPh)(CC≡CPh). In contrast, the reaction of the same silylene with two equivalents diphenylacetylene afforded a silole in a [2+2+1] cycloaddition reaction.
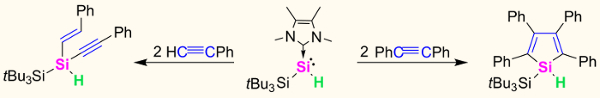
Both reaction mechanisms were studied in detail by DFT calculations and isotopic labeling experiments revealing that the NHC plays a major role in supporting the formation of zwitterionic intermediates and transition states.
- Reaction of an N-Heterocyclic Carbene-Stabilized Silicon(II) Monohydride with Alkynes: [2+2+1] Cycloaddition versus Hydrogen Abstraction,
Carsten Eisenhut, Tibor Szilvási, Nora C. Breit, Shigeyoshi Inoue,
Chem. Eur. J. 2014.
DOI: 10.1002/chem.201405303
![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
