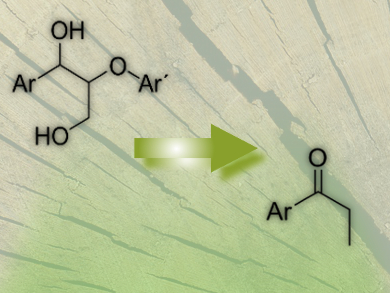
The biopolymer lignin (pictured below) is an attractive feedstock for the production of chemicals and fuels. Although the chemical structure of lignin is complex, reductive and oxidative methodologies are known to promote the activity of the β-O-4′-glycerolaryl ether linkage. These methods generally proceed through C–C bond cleavage and result in degradation of the propylbenzene skeleton. However, in some cases conservation of the alkyl chain is required to selectively generate the aryl ethyl ketone. This selective oxidation, without C–C bond cleavage, represents a major challenge in lignin chemistry.
Joseph S. M. Samec, Uppsala University, Sweden, and colleagues have developed a mild and selective oxidation of the benzylic alcohol in dimeric lignol model compounds that proceeds without C–O or C–C bond cleavage or γ-alcohol dehydrogenation. The resulting compounds are highly valuable for fine chemical synthesis. The reaction utilizes a recyclable Pd/C catalyst in environmentally friendly solvents such as ethyl acetate or ethanol under mild conditions with air as an oxidant. Interestingly, the key to the success of this reaction was found to be the addition of catalytic amounts of glucose, which is proposed to inhibit catalyst deactivation by stabilization of the palladium catalyst, preventing aggregation.
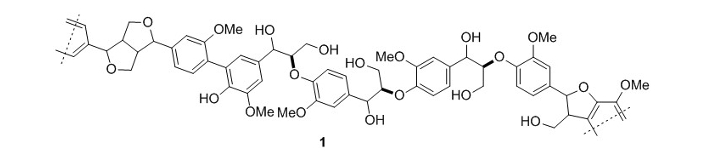
Along with the one-pot synthesis of valuable compounds, the combination of mild reaction conditions, air as an oxidant, and the use of green solvents makes this reaction interesting from an economical and environmental viewpoint.
- Selective Aerobic Benzylic Alcohol Oxidation of Lignin Model Compounds: Route to Aryl Ketones,
Monali Dawange, Maxim V. Galkin, Joseph S. M. Samec,
ChemCatChem 2015.
DOI: 10.1002/cctc.201402825