Sustainable methods for producing energy are in high demand as a result of the pressures of climate change and limitations to current resources. The production of solar fuels such as H2 by the splitting of H2O constitutes an appealing option for sustainable energy conversion. Thus the development of artificial water oxidation catalysts (WOCs) has become an important research field. Considerable progress has been made during the last couple of years on the construction of WOCs based on Ru, Ir, Mn, Fe, Co, and Cu, yet the mechanistic description associated with such catalysts is usually not well defined. To facilitate the design of more efficient future catalysts, it is important to fully understand the mechanism by which H2O is oxidized by artificial WOCs.
Markus Kärkäs, Rong-Zhen Liao, Björn Åkermark, and co-workers, Stockholm University, Sweden, and Huazhong University of Science and Technology, Wuhan, China, provide an insight into this mechanistic process by using a dinuclear Ru-based WOC.
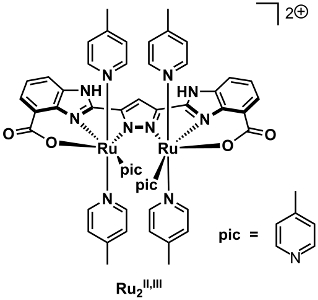
The catalytic action of the designed Ru complex was studied by the combined use of high-resolution mass spectrometry, electrochemistry, and quantum chemical calculations. Based on the results, it is suggested that the designed ligand scaffold in the Ru complex has a non-innocent behavior, in which metal–ligand cooperation is an important part during the four-electron oxidation of H2O. This feature is vital for the observed catalytic efficiency and highlights that the preparation of catalysts housing non-innocent molecular frameworks could be a general strategy for accessing efficient catalysts for activation of H2O.
- A Dinuclear Ruthenium-Based Water Oxidation Catalyst: Use of Non-Innocent Ligand Frameworks for Promoting Multi-Electron Reactions,
Tanja M. Laine, Markus D. Kärkäs, Rong-Zhen Liao, Per E. M. Siegbahn, Björn Åkermark,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201406613