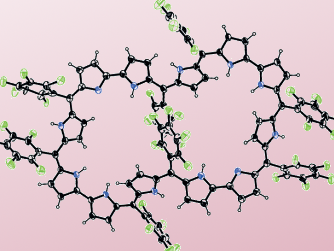
Hückel aromatic molecules must be planar and obey the 4n+2 rule. Expanded porphyrins may be candidates for aromatic species larger than those reported thus far. However, as the ring size becomes larger, porphyrins tend to lose molecular planarity and take on twisted conformations. This is mainly due to the increasing conformational flexibility and the increasing importance of intramolecular hydrogen bonding. As a consequence, planar expanded porphyrins possessing aromatic characters are rare and structurally well-characterized examples are even more limited.
Dongho Kim, Yonsei University, Seoul, South Korea, Atsuhiro Osuka, Kyoto University, Japan, and colleagues oxidized the nonaromatic [52]dodecaphyrin(1.1.0.1.1.0.1.1.0.1.1.0) to the corresponding [50]dodecaphyrin and the [48]dodecaphyrin. The [50]dodecaphyrin was further deprotonated to the tetraprotonated dodecaphyrin. The compounds were characterized with UV-Vis and NMR spectroscopy as well as cyclic voltammetry.
With 50 π-electrons, the [50]dodecaphyrin and the tetraprotonated dodecaphyrin are the largest Hückel aromatic molecules reported to date.
- Stable [48]-, [50]-, and [52]Dodecaphyrins(1.1.0.1.1.0.1.1.0.1.1.0): The Largest Hückel Aromatic Molecules,
Takanori Soya, Woojae Kim, Dongho Kim, Atsuhiro Osuka,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201500650