The family of epipolythiodiketopiperazine (ETP) natural products consists of over 200 members possessing a wide diversity of structures and biological activity. Recently, the subgroup of 6–5–6–5–6-membered ETPs has gained substantial attention. The most elusive members of this group, the scabrosin esters, were isolated in 1978 from the lichen Xanthoparmelia scabrosa. They display potent anticancer activity at the nanomolar range. However, despite all the efforts that have been invested into accessing these complex structures, no synthesis of scabrosin diacetate and its related esters has as of yet been reported.
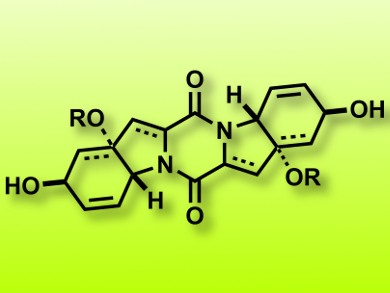
Erick M. Carreira and co-workers, Eidgenössische Technische Hochschule (ETH) Zurich, Switzerland, have developed a concise and scalable route to the core structure of 6–5–6–5–6-fused ETP natural products (pictured). The strategy is different from previous approaches in that a two-directional approach is followed: Bidirectional opening of bis(oxabicyclo[2.2.1]heptane) allows the rapid construction of the core bis(tetrahydroindole) structure, which is then functionalized on both symmetry equivalent sides to give access to a wide diversity of functionalized 6–5–6–5–6-membered diketopiperazines.
Although it was not possible to transform the late-stage intermediate bis(tetrahydroindole) core into scabrosin diacetate, these results should encourage the future use of this strategy to access highly functionalized diketopiperazines for structure–activity relationship (SAR) studies or natural product synthesis.
- A Unified Strategy to 6–5–6–5–6-Membered Epipolythiodiketopiperazines: Studies towards the Total Synthesis of Scabrosin Diacetate and Haematociny,
Hannes F. Zipfel, Erick M. Carreira,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201500918
![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
