Artificial photosynthesis is a strategy for converting light energy into storable chemical fuels in a single process. In one approach, water is split directly into oxygen and hydrogen, the latter of which is highly efficient for energy storage and conversion. Overall water-splitting processes may be based on separate water oxidation and reduction catalysts, which are often polyoxometalates (POMs). This large family of metal clusters displays manifold structural modifications and diverse chemical reactivity.
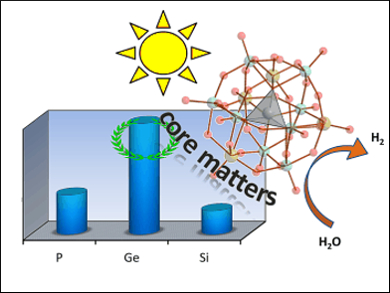
To better understand POM-based water reduction catalysts, Greta Patzke, University of Zurich, Switzerland, and colleagues have probed the structure-activity relationships of these multifaceted materials. Nickel-substituted Keggin-type POMs [Ni(H2O)XW11O39]n- (X = P, Ge, Si) were investigated as water reduction catalysts with [Ru(bpy)3]2+ as a photosensitizer.
The heteroatom X was found to exert a strong influence on the catalytic activity, and the Ge-containing POM displayed the best performance. However, X does not affect the energy level of the lowest unoccupied molecular orbital (LUMO), and the photochemical steps differ considerably from the electrochemical pathways. This finding is different from previously described structure-activity relationships of POM catalysts and is an unexpected turn on the path to optimized cluster catalysts for photochemical water reduction.
- Nickel-Containing Keggin-Type Polyoxometalates as Hydrogen Evolution Catalysts: Photochemical Structure-Activity Relationships,
Kim von Allmen, René Moré, Rafael Müller, Joaquín Soriano-López, Anthony Linden, Greta R. Patzke,
ChemPlusChem 2015.
DOI: 10.1002/cplu.201500074
Greta Patzke has been featured in ChemPlusChem’s Early Career Series.