Human neutrophil elastase (HNE) is a highly active enzyme that breaks down mechanically important protein structures of the body’s own cellular matrix (proteins like elastin and collagen), as well as foreign proteins such as those of the cell walls of Gram-negative bacteria. An imbalance in HNE activity can lead to many inflammatory diseases such as chronic obstructive pulmonary disease (COPD), bronchiectasis (BE), pulmonary arterial hypertension, and pulmonary fibrosis.
Only few of the various HNE inhibitors developed so far have had an overall profile suitable for clinical testing. Indeed, combining drug potency with selectivity is generally difficult, particularly for small-molecule protease inhibitors.
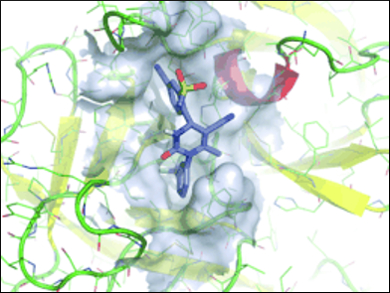
Franz von Nussbaum, Bayer HealthCare, Berlin, Germany, and colleagues worked their way around this problem and discovered a new, highly potent and selective HNE inhibitor (BAY 85-8501) with an unprecedented locked bioactive conformation. This means the molecule is imbued with a very encouraging combination of selectivity and potency. BAY 85-8501 is currently under investigation in a safety and efficacy trial in patients with BE.
- Freezing the Bioactive Conformation to Boost Potency: The Identification of BAY 85-8501, a Selective and Potent Inhibitor of Human Neutrophil Elastase for Pulmonary Diseases,
Franz von Nussbaum, Volkhart M.-J. Li, Swen Allerheiligen, Sonja Anlauf, Lars Bärfacker, Martin Bechem, Martina Delbeck, Mary F. Fitzgerald, Michael Gerisch, Heike Gielen-Haertwig, Helmut Haning, Dagmar Karthaus, Dieter Lang, Klemens Lustig, Daniel Meibom, Joachim Mittendorf, Ulrich Rosentreter, Martina Schäfer, Stefan Schäfer, Jens Schamberger, Leila A. Telan, Adrian Tersteegen,
ChemMedChem 2015.
DOI: 10.1002/cmdc.201500131