Long-Standing Puzzle About Small Molecule Solved
Does ethylenedione exist or not? This controversy about the tiny OCCO molecule, which is formally a dimer of carbon monoxide, has been quietly continuing for about a century. American researchers have now presented conclusive experimental proof for the existence of this mysterious compound in the journal Angewandte Chemie. Using photodetachment photoelectron spectroscopy, they were able to make ethylenedione and to spectroscopically characterize the electronic states of the molecule.
Despite the apparently simple structure of O=C=C=O, in which the atoms are all linked by double bonds, the molecule has been hard to track down. It was first postulated in 1913 as an intermediate in the reaction of oxalyl bromide with mercury to make carbon monoxide (CO). In the 1940s, it was claimed to be the active component of Glyoxylide, a purported antidote for a long list of afflictions, from exhaustion to cancer, which was soon revealed to be a complete fraud. The synthesis of OCCO has been tried for decades, but the molecule never even turned up as a short-lived intermediate.
Using Photoelectron Spectroscopy to Study Ethylenedione
Andrei Sanov and his students Andrew Dixon and Tian Xue at the University of Arizona, Tucson, USA, have now finally proven the existence of OCCO. Using a special reaction involving negatively charged oxygen radicals, they converted glyoxal (C2H2O2) to an ethylenedione anion, which bears a single negative charge. They then used photodetachment photoelectron spectroscopy to study the neutral species that results from the removal of the excess charge from this anion.
In this technique, injection of a defined amount of energy in the form of a photon is used to “shoot” the extra electron away from the anion. The energy of the electrons being released in relation to the incoming photon energy gives an energy spectrum that makes it possible to draw conclusions about the energy states and modes of vibration of the neutral molecule which is formed during this spectroscopic examination.
Bound Triplet State with a Nanosecond Lifetime
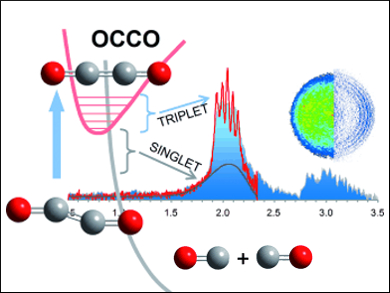
The photoelectron spectra indicate two states, which the researchers conclude are the bound triplet and a dissociative singlet state of OCCO. They are in agreement with the theoretical predictions and quantum chemical calculations.
The lowest-energy bound state of OCCO is a linear triplet state. This means that there are two unpaired electrons, making the compound a biradical similar to the oxygen molecule O2. This triplet state can easily change to a singlet state, which has paired electrons but also a non-bonding character. This causes the molecule to dissociate rapidly into two CO molecules and explains the elusive nature of OCCO. The triplet-to-singlet transition happens very fast from a human perspective – in a fraction of a nanosecond. This is, however, a long lifetime in the molecular realm, and it makes triplet ethylenedione a chemical identity as a transient molecule and a reactive intermediate.
- Spectroscopy of Ethylenedione,
Andrew R. Dixon, Tian Xue, Andrei Sanov,
Angewandte Chemie International Edition 2015.
DOI: 10.1002/anie.201503423