Since its development by Heck in the 1970s, aminocarbonylation of aryl halides catalyzed by transition metals (particularly Pd) has been a widely used strategy for accessing aromatic amides. The amides in turn are a common intermediate in organic synthesis. Given the high cost of transition metal catalysts, there is an interest in developing alternate methods.
Ilhyong Ryu and colleagues, Osaka Prefecture University, Japan, have developed a procedure for aryl aminocarbonylation with CO that requires no catalyst at all. Inspired by developments in aryl radical carbonylation, Professor Ryu’s group predicted that aminocarbonylation or aryl iodides could be carried out photochemically. Under irradiation from a 500 W xenon lamp, photogenerated aryl radicals add to CO to form acyl radicals, which can in turn be trapped by amines.
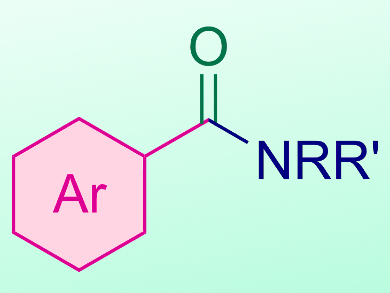
By varying the substituents on the aryl iodide, as well as the identity of the amine, the team synthesized a wide range of aryl amide products in yields up to 89 %. This simple procedure could pave the way for the development of further metal-free carbonylation reactions.
- Photoinduced Aminocarbonylation of Aryl Iodides,
Takuji Kawamoto, Aoi Sato, Ilhyong Ryu,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201503164


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
